Abstract
This study was conducted to evaluate the microtubule distribution following control of nuclear remodeling by treatment of bovine somatic cell nuclear transfer (SCNT) embryos with caffeine or roscovitine. Bovine somatic cells were fused to enucleated oocytes treated with either 5 mM caffeine or 150 µM roscovitine to control the type of nuclear remodeling. The proportion of embryos that underwent premature chromosome condensation (PCC) was increased by caffeine treatment but was reduced by roscovitine treatment (p < 0.05). The microtubule organization was examined by immunostaining β- and γ-tubulins at 15 min, 3 h, and 20 h of fusion using laser scanning confocal microscopy. The γ-tubulin foci inherited from the donor centrosome were observed in most of the SCNT embryos at 15 min of fusion (91.3%) and most of them did not disappear until 3 h after fusion, regardless of treatment (82.9-87.2%). A significantly high proportion of embryos showing an abnormal chromosome or microtubule distribution was observed in the roscovitine-treated group (40.0%, p < 0.05) compared to the caffeine-treated group (22.1%). In conclusion, PCC is a favorable condition for the normal organization of microtubules, and inhibition of PCC can cause abnormal mitotic division of bovine SCNT embryos by causing microtubule dysfunction.
In somatic cell nuclear transfer (SCNT), the nuclear remodeling of the transferred nucleus might play an important role in the subsequent development of SCNT embryos [2,13], and this may be relevant to the microtubule assembly of the donor nucleus. Microtubules are major cytoskeletal elements found in mammalian oocytes and have a crucial role in meiotic/mitotic events, such as chromosomal movement and cellular division. Accurate cytoskeletal movement is essential for normal embryo development. Microtubules are composed of α- and β-tubulins, which are organized around a microtubule-organizing center (MTOC). In animal cells, a centrosome comprising a pair of centrioles surrounded by pericentriolar material acts as an MTOC. In mammals, the centrosome plays a vital role both in microtubule nucleation and in establishing spindle bipolarity [9,10]. γ-tubulin, the permanently centrosome-associated microtubule-nucleating protein, is an essential centrosome component associated with the pericentriolar region [24] and nucleates the centriolar microtubules [18,20,30]. Thus, the γ-tubulin foci that are located in the spindle poles are considered to represent centrosomes [23].
Cellular events occurring within hours of SCNT are important for the survival of cloned embryos. Nevertheless, little is known about the nuclear and cytoplasmic changes associated with the integration of donor cells into the recipient cytoplasm [28]. During SCNT, the meiotic spindle complex of an oocyte is removed, and the centrosome of a donor cell is introduced into the enucleated recipient cytoplasm. Moreover, the fate of the transferred centrosome might be affected by the type of nuclear remodeling in the transferred donor cells.
In SCNT, the level of maturation promoting factor (MPF) activity in the recipient oocyte can affect the remodeling type of a transferred nucleus, which can in turn affect the reprogramming of SCNT embryos, including steps involving blastocyst development and the methylation status [13,16,19]. The MPF activity can be controlled by p34cdc2 kinase-activity regulators, caffeine [11], and roscovitine [7]. Caffeine increases the MPF activity [11], whereas roscovitine decreases the MPF activity of recipient oocytes [7].
The present study was carried out to evaluate the microtubule distribution following regulation of the nuclear remodeling type by caffeine or roscovitine treatments in bovine SCNT embryos.
Bovine cumulus-oocyte complexes (COCs) were aspirated from follicles (2- to 7-mm diameter) of ovaries and selected based on their morphology. They were washed in Tyrode's lactate-Hepes buffer containing 0.1% (w/v) polyvinyl alcohol (PVA; Sigma, USA). The culture medium for in vitro maturation was Tissue Culture Medium 199 (TCM199; Gibco-BRL, USA) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL, USA), 0.02 U/mL follicle-stimulating hormone (Sigma, USA), 1 µg/mL estradiol (Sigma, USA), and 50 µg/mL gentamicin (Sigma, USA). Ten COCs were transferred into 50 µL droplets of maturation medium overlaid with paraffin oil and cultured for 20-22 h at 39℃, 5% CO2 in air.
After in vitro maturation of COCs, the cumulus cells were removed by vortexing for 5 min in phosphate-buffered saline (PBS) supplemented with 0.1% (w/v) hyaluronidase (Sigma, USA) and 0.1% (w/v) PVA (Sigma, USA). Before the enucleation, oocytes were cultured in TCM199 containing 0.4 µg/mL demecolcine for 40 min to extrude their metaphase II (MII) chromosome mass [29]. The enucleation of oocytes was carried out by removing the MII chromosome mass and the 1st polar body in Hepes-buffered TCM199 (Gibco-BRL, USA) supplemented with 3 mg/mL bovine serum albumin (BSA; Sigma, USA) and 5 µg/mL cytochalasin B (CB; Sigma, USA). Prior to nuclear transfer, enucleated oocytes were cultured in TCM199 (Gibco-BRL, USA) containing 3 mg/mL BSA and either 5 mM caffeine (Sigma, USA) for 6 h or 150 µM roscovitine (Sigma, USA) for 1.5 h [19]. In our separate study, treatments with caffeine and roscovitine did not affect the development of bovine parthenogenetic embryos [19].
SCNT was carried out in Hepes-buffered TCM199 supplemented with 3 mg/mL BSA and 5 µg/mL CB (Sigma, USA). Ear skin fibroblast cells (4~6 passaged) from a Korean native cow were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, USA) supplemented with 10% FBS (Gibco-BRL, USA) and 50 µg/mL gentamicin (Sigma, USA) for 2~3 days, and then cultured for 5 days in DMEM/F12 containing 0.5% FBS. Before injection, the cells were trypsinized and then centrifuged in TCM199 medium supplemented with 3 mg/mL BSA. Subsequently, a donor cell was transferred into the perivitelline space of enucleated recipient oocytes at the same maturational age (at around the 27~28 h of IVM) for all types of recipient oocytes. For the caffeine- or roscovitine-treated enucleated oocytes, donor cells were transferred within 0.5 h after release from these chemicals.
Reconstructed oocytes were electrically fused. They were placed between wire electrodes (1 mm apart) of a fusion chamber that was overlaid with 0.3 M mannitol solution supplemented with 0.1 mM MgSO4, 0.05 mM CaCl2 and 0.1% BSA. A single direct-current pulse of 1.3 kV/cm was applied for 30 µsec using a BTX Electro Cell Manipulator 200 (BTX, USA). After fusion treatment, the fused oocytes were activated using 10 µM Ca-ionophore (A23187; Sigma, USA) for 5 min and subsequently cultured in CR1aa [21] containing 2 mM 6-dimethylaminopurine (Sigma, USA) for 4 h.
The SCNT embryos were whole mounted 1.5 h after fusion to assess the type of remodeling of the transferred nucleus. The SCNT embryos were mounted on slides, fixed with a mixture of ethanol and acetic acid (3 : 1) for 48 h, then stained with 1% aceto-orcein for 5 min and washed in 25% (v/v) aceto-glycerol. The nuclear morphology was observed under a phase-contrast microscope (×400). Transferred nuclei showing a chromosome plate or a condensed chromatin clump were classified as PCC and those displaying a pronucleus (PN)-like structure without nuclear envelop breakdown (NEBD) were classified as non-PCC (NPCC) [3].
In vitro oocytes matured for 22 h were inseminated with frozen-thawed spermatozoa (2 × 106 spermatozoa/mL) in a 50 µL drop of BO medium [1] containing 5 mM caffeine (Sigma, USA), 10 µg/mL heparin (Sigma, USA) and 3 mg/mL BSA at 39℃, 5% CO2 in air for 8 h.
After activation or insemination culture, the SCNT embryos and IVF embryos were further cultured in CR1aa containing 3 mg/mL BSA and 50 µg/mL gentamicin at 39℃, 5% CO2 in air for 16~20 h. In one experiment, IVF embryos were treated with caffeine for 6 h or roscovitine for 1.5 h at the beginning of culture to evaluate the effects of chemicals on the microtubule distribution of embryos at the first mitotic phase.
For the confocal microscopy, fused embryos were fixed 15 min, 3 h and 20 h after fusion with 3.7% (w/v) paraformaldehyde (Sigma, USA) in PBS for 30 min at room temperature. IVF embryos were fixed at 28 h of insemination. MII oocytes were also fixed. Fixed oocytes and embryos were stored in PBS containing 3 mg/mL BSA and 0.02% (w/v) sodium azide for 1 week at 4℃. They were then permeabilized by transferring into PBS containing 0.1% (w/v) Triton X-100, 3 mg/mL BSA and 0.02% (w/v) sodium azide for 50 min at 39℃. After several washing steps with PBS containing 0.01% (w/v) Triton X-100, they were incubated in a blocking solution with PBS containing 150 mM glycine, 3 mg/mL BSA and 0.02% (w/v) sodium azide for 50 min at 39℃. Microtubule localization was conducted using a mouse monoclonal antibody against β-tubulin (Sigma, USA) and rabbit monoclonal antibody against γ-tubulin (Sigma, USA). Oocytes and embryos were incubated for 1 h at 39℃ with 1 : 200 (w/v) dilutions of anti-β-tubulin and anti-γ-tubulin. Primary antibodies were detected using Alexa-488 goat anti-mouse IgG and Alexa-546 goat anti-rabbit IgG (Molecular Probes, USA) at 1 : 100 (w/v) dilutions. Oocytes and embryos were incubated with secondary antibody for 40 min at 39℃. DNA was stained with 2 µg/mL of Hoechst 33342 for 40 min at 39℃. Stained oocytes and embryos were then mounted on slides in Vecta-Shield antifade (Vector Laboratories, USA) under a coverslip. The samples were examined with a laser-scanning confocal microscope (Zeiss LSM 510; Jena, Germany).
Embryos were fixed by the air-drying method in order to analyze the chromosome constitutions. After 12 h of activation, one-cell stage SCNT embryos were incubated for 6~8 h in CR1aa containing 0.4 µg/mL of nocodazole (Sigma, USA) in order to arrest cleavage division at the mitotic phase. They were then treated for 10 min with a hypotonic solution of 0.9% trisodium citrate. The embryos were fixed for 10 min with a mixture of methanol, acetic acid, and distilled water (5 : 1 : 4), mounted on a slide, and further fixed by the drop application of a mixture of methanol and acetic acid (3 : 1), followed by soaking in the same fixative. The samples were rinsed in a mixture of methanol, acetic acid, and distilled water (3 : 4 : 1) to remove cytoplasmic debris, stained for 10 min with 5% Giemsa, and the chromosome constitutions of the embryos were assessed.
In the control group, 72.1% (88/122) of the SCNT embryos showed PCC. Caffeine treatment resulted in a higher occurrence of PCC (81.7%, 98/120, p < 0.05), whereas roscovitine treatment resulted in a lower occurrence of PCC (24.2%, 29/120, p < 0.05).
The microtubule distribution in bovine SCNT embryos at 15 min and the effects of caffeine and roscovitine on the distribution at 3 h after fusion are indicated in Figs. 1 and 2. The γ-tubulin focus was not observed in matured MII oocytes (Fig. 1A) and in a small number (4/46, 8.7%, Fig. 2) of SCNT embryos at 15 min after fusion (Fig. 1B). The γ-tubulin focus inherited from the donor centrosome (Figs. 1C and D) was observed in the majority of SCNT embryos at 15 min of fusion (91.3%, Fig. 2), and most of these γ-tubulin foci did not disappear from their cytoplasm until 3 h after fusion, regardless of treatment (82.9~87.2%, Fig. 2).
Microtubule distributions in the SCNT embryos at the first mitotic phase are shown in Fig. 3. One or two γ-tubulin foci were formed around the PN-like structure of SCNT embryos in the interphase (Fig. 3A). At the mitotic phase, the spindle microtubule was completely formed from the γ-tubulin foci (Figs. 3B and C). The spindle microtubule was maintained until the anaphase (Fig. 3D), but the γ-tubulin foci had disappeared at the ana-telophase in SCNT embryos (Fig. 3E). γ-Tubulin focus was detected at the two-cell stage of SCNT embryos (Fig. 3F). This pattern was similar to that of IVF embryos (not shown).
Microtubule organization in the first mitotic phase of bovine SCNT embryos following treatment with caffeine or roscovitine is summarized in Table 1. There was no difference in the proportion of embryos showing γ-tubulin focus among the three groups. Various types of embryos showing an abnormal microtubule distribution were detected in the first mitotic phase of SCNT embryos (Fig. 4). The proportion of embryos showing an abnormal microtubule distribution was significantly increased (p < 0.05) in the roscovitine-treated group (40.0%, 28/70) compared with the caffeine-treated group (22.1%, 15/68). There was no difference in the types of abnormal microtubule distribution among the treatment groups (not shown). There were no influences of caffeine and roscovitine on the microtubule distribution in the first mitotic phase of IVF embryos (data not shown).
The chromosome constitutions of SCNT embryos at the 1-cell stage did not differ among the control and caffeine- and roscovitine-treated groups (Table 2). The proportion of embryos with a normal diploid chromosome set was 71.1 to 77.6%, regardless of treatments. Small numbers of haploid or tetraploid chromosome sets were noted. A similar frequency of aneuploid chromosome sets was also observed in all groups of SCNT embryos (16.3 to 26.7%).
In nuclear transfer studies, the MPF in the recipient cytoplasm can regulate the nuclear remodeling type of the donor nucleus [2,5]. In this study we used caffeine and roscovitine to regulate the MPF activity of the recipient cytoplasm. We did not evaluate the MPF activity of recipient oocytes; however, caffeine and roscovitine can successfully regulate both the MPF activity [7,11,13,19] and the subsequent nuclear remodeling type after transfer of donor cells [7,13]. The nuclear remodeling type of a transferred nucleus affects the subsequent development of nuclear transfer embryos [2,5,13,19].
The remodeling of the centrosome in SCNT embryos is important because centrosomes are introduced along with the donor nucleus and carry out many critical functions that are normally carried out by the sperm centrosomes. In mammal cells, γ-tubulin is an essential component associated with the pericentriolar region of the centrosome [24]. The γ-tubulin was reported to participate in the spindle assembly of bovine SCNT embryos, to be located in the spindle poles, and, when associated with interphase nuclei, to represent reconstituted centrosomes [23]. In this study, γ-tubulin foci were observed in all of the cell cycle phases in SCNT embryos except the ana-telophase. The result in SCNT embryos showed that the centrosomes of donor cells are normally passed on to recipient oocytes. At 20 h after fusion, most of the SCNT embryos at the first mitotic stage had one or two (duplicated) γ-tubulin foci, which were classified as normal, whereas some embryos had more than three γ-tubulin foci. Two-cell embryos had two, three, or four γ-tubulin foci, which were ascertained as normal. The duration of the second cell cycle in bovine embryos is very short-about 9 h. Therefore, most two-cell embryos are expected to enter the S-phase of the second cell cycle immediately after cleavage [14], and may have three (partially duplicated) or four (duplicated) γ-tubulin foci. In this study, the γ-tubulin foci disappeared in the ana-telophase in SCNT embryos. Zhong et al. [31] reported that faint γ-tubulin staining was located at the spindle poles, although centrosomes were not detected at the telophase of SCNT porcine embryos. In another study, two centrosomes, presumably produced by splitting, were detected at the anaphase and telophase of the first mitotic stage in SCNT bovine embryos [6]. We cannot predict whether the lack of γ-tubulin foci at the ana/telophase transition stage in this study is because of the faint γ-tubulin staining or the dispersion of γ-tubulin foci.
The microtubule localization and nuclear progression of SCNT embryos were visualized by immunostaining of β- or γ-tubulin and DNA. By the SCNT procedure, the centrosome (γ-tubulin focus) of a donor cell is introduced into a recipient cytoplasm and is located in association with a PN-like structure in bovine [23]. In our study, the γ-tubulin focus of the donor cell was observed near the transferred nucleus of bovine SCNT embryos immediately after fusion and was detected 3 h after fusion also. Studies on the mouse SCNT showed that many acentrosomal γ-tubulin foci generated in cytoplasm disappeared after activation [17] and that a donor centrin, which is a basic component of the centrosome [22], is also degraded in activated ooplasm [32]. It is suggested that the pattern of centrosome inheritance during SCNT may differ among species. In our study, the transition pattern of the donor centrosome (γ-tubulin focus) was not different among the control, caffeine and roscovitine groups. It is hence suggested that the transition pattern of the centrosome from a donor cell is not affected by the type of nuclear remodeling.
A high frequency of abnormal chromosome and microtubule distribution was observed in the roscovitine-treated group. It was reported that roscovitine treatment can induce some morphological modifications of cytoplasmic components such as mitochondria and cytoskeletons of matured oocytes [8,15]. In our study, there was no influence of roscovitine on the microtubule distribution in the first mitotic phase of IVF embryos. Furthermore, in our separate study, there were no adverse effects on the developmental capability of parthenogenetic embryos treated with roscovitine under the same conditions as in the present study [19]. Therefore, it is possible that the NPCC, but not roscovitine treatment itself, could affect the cytoplasmic components of the transferred donor cell, which could in turn induce an abnormal microtubule formation due to the incomplete remodeling of the transferred centrosome. It was previously suggested that NEBD is a critical step in the reprogramming of somatic cell nuclei in its allowing of the direct interaction of chromosomes with cytoplasmic factors in nonactivated oocytes [12,25,27]. It was also suggested that MPF activity itself does not directly regulate the reprogramming of somatic cell nuclei, but that the exposure of donor chromosomes to oocyte cytoplasm, i.e. the induction of NEBD and PCC, is important for the occurrence of essential events that facilitate the reprogramming of the donor nuclei at the G0/G1 stage [26]. It appears that the increased abnormal microtubules might be responsible for the low in vitro development of NPCC-induced SCNT embryos [13,19].
In bovine SCNT, some PCC-induced embryos harbored multiple chromatin clumps and extruded a polar body after activation treatment, and may have resulted in aneuploid chromosome constitutions [4]. In this study, we did not address polar body extrusion, but approximately 22~29% of embryos manifested abnormal ploidy. We cannot, at this point, be sure as to whether the abnormal ploidy resulted from PCC or from NPCC, as similar proportions of embryos with abnormal ploidy were observed regardless of the treatments administered. It appears that the abnormal microtubule distributions of the SCNT embryos after treatment with roscovitine do not influence the chromosome constitutions of the SCNT embryos at the 1-cell stage.
In conclusion, the nuclear remodeling type, which is regulated by caffeine or roscovitine treatments, appears to be related to microtubule function in the early embryonic stages of SCNT embryos. The PCC is a more favorable condition for the normal organization of microtubules, and inhibition of PCC may lead to incessant abnormal mitotic division of bovine SCNT embryos by causing dysfunction of their microtubules, which can affect their subsequent development [19].
Figures and Tables
Fig. 1
Pattern of centrosome transition and its localization in bovine somatic cell nuclear transfer (SCNT) embryos. The transiting centrosome and its localization in bovine SCNT embryos were examined 15 min after fusion. (A) Metaphase II (MII) spindle in an MII oocyte. (B-D) SCNT embryos with no (B), one (C) and two γ-tubulin foci (D) are seen near the nucleus. Insets indicate DNA (d; blue), β-tubulin (b; green), γ-tubulin (g; red), and merged (m) images. Scale bar =50 µm.
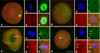
Fig. 2
Transition patterns of the donor centrosome in bovine SCNT embryos. The number of γ-tubulin foci (□ one, ░ two, ▨ more than three, and ▪ none) in bovine SCNT embryos was examined by laser scanning confocal microscopy 15 min and 3 h after fusion. The total numbers of embryos in the control at 15 min, control at 3 h, and the caffeine and roscovitine groups, respectively, were 46, 35, 39 and 39.
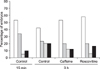
Fig. 3
Nuclear progression and microtubule organization in the first mitotic phase of bovine SCNT embryos. Nuclear progression and microtubule distribution in the different phases of the first mitosis of SCNT embryos were examined 20 h after fusion. (A) Interphase. Two γ-tubulin foci are seen around a pronucleus-like structure. (B) Prophase. (C) Prometaphase. (D) Metaphase. (E) Ana-telophase. γ-tubulin foci were not detected. (F) Two-cell stage, One γ-tubulin focus is seen around the nucleus of both blastomeres. Insets indicate DNA (d; blue), β-tubulin (b; green), γ-tubulin (g; red; arrows), and merged (m) images. Scale bar = 50 µm.
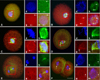
Fig. 4
Abnormal nuclear and microtubule configuration in the first mitotic phase of bovine SCNT embryos. Microtubule distribution in the SCNT embryos was examined 20 h after fusion. (A) Multiple and multipolar γ-tubulin foci were organized near the nucleus of SCNT embryos. (B) Chromosomes were condensed but mitotic spindles were not organized. (C) Mitotic spindles were organized without any spindle poles. (D) Mitotic spindles were organized from multiple poles. (E) Two γ-tubulin foci were seen near the mitotic spindle but did not connect with them. (F) Three mitotic spindles were organized without any spindle poles. (G) A cytoplasmic microtubule network from a focus (within square) was organized at the opposite pole of the nucleus. (H) Cytoplasmic fragment without a nucleus. Insets indicate DNA (d; blue), β-tubulin (b; green), γ-tubulin (g; red), and merged (m) images. Scale bar = 50 µm.
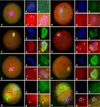
Table 1
Organization of a γ-tubulin focus in the first mitotic phase of bovine somatic cell nuclear transfer (SCNT) embryos

Abnormal, embryos belonging to an abnormal category, as represented in Fig. 4. Nuclear progression and microtubule organization of SCNT embryos were examined by confocal laser microscopy 20 h after fusion. a,bValues with different superscripts are significantly different (p < 0.05).
Acknowledgments
We thank Mr. Seung-Hae Kwon of the Korea Basic Science Institute, Chuncheon, Korea, for his expert assistance with confocal microscopy. This work was supported by a Korea Research Foundation Grant, funded by the Korean Government (MOEHRD) (KRF-2006-311-F00018).
References
1. Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod. 1975. 12:260–274.
2. Cheong HT, Takahashi Y, Kanagawa H. Birth of mice after transplantation of early cell-cycle-stage embryonic nuclei into enucleated oocytes. Biol Reprod. 1993. 48:958–963.

3. Cheong HT, Takahashi Y, Kanagawa H. Relationship between nuclear remodeling and subsequent development of mouse embryonic nuclei transferred to enucleated oocytes. Mol Reprod Dev. 1994. 37:138–145.

4. Choi JY, Kim CI, Park CK, Yang BK, Cheong HT. Effect of activation time on the nuclear remodeling and in vitro development of nuclear transfer embryos derived from bovine somatic cells. Mol Reprod Dev. 2004. 69:289–295.

5. Collas P, Robl JM. Relationship between nuclear remodeling and development in nuclear transplant rabbit embryos. Biol Reprod. 1991. 45:455–465.

6. Dai Y, Wang L, Wang H, Liu Y, Li N, Lyu Q, Keefe DL, Albertini DF, Liu L. Fate of centrosomes following somatic cell nuclear transfer (SCNT) in bovine oocytes. Reproduction. 2006. 131:1051–1061.

7. Ito J, Hirabayashi M, Kato M, Takeuchi A, Ito M, Shimada M, Hochi S. Contribution of high p34cdc2 kinase activity to premature chromosome condensation of injected somatic cell nuclei in rat oocytes. Reproduction. 2005. 129:171–180.

8. Ju JC, Tsay C, Ruan CW. Alterations and reversibility in the chromatin, cytoskeleton and development of pig oocytes treated with roscovitine. Mol Reprod Dev. 2003. 64:482–491.

10. Kellogg DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994. 63:639–674.

11. Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Aoki F, Tojo H, Toyoda Y. Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biol Reprod. 2000. 63:715–722.

12. Kim JM, Ogura A, Nagata M, Aoki F. Analysis of the mechanism for chromatin remodeling in embryos reconstructed by somatic nuclear transfer. Biol Reprod. 2002. 67:760–766.

13. Kwon DJ, Park CK, Yang BK, Cheong HT. Control of nuclear remodelling and subsequent in vitro development and methylation status of porcine nuclear transfer embryos. Reproduction. 2008. 135:649–656.

14. Lequarre AS, Marchandise J, Moreau B, Massip A, Donnay I. Cell cycle duration at the time of maternal zygotic transition for in vitro produced bovine embryos: effect of oxygen tension and transcription inhibition. Biol Reprod. 2003. 69:1707–1713.

15. Lonergan P, Faerge I, Hyttel PM, Boland M, Fair T. Ultrastructural modifications in bovine oocytes maintained in meiotic arrest in vitro using roscovitine or butyrolactone. Mol Reprod Dev. 2003. 64:369–378.

16. Ma W, Zhang D, Hou Y, Li YH, Sun QY, Sun XF, Wang WH. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod. 2005. 72:373–383.

17. Miki H, Inoue K, Ogonuki N, Mochida K, Nagashima H, Baba T, Ogura A. Cytoplasmic asters are required for progression past the first cell cycle in cloned mouse embryos. Biol Reprod. 2004. 71:2022–2028.

18. Moudjou M, Bordes N, Paintrand M, Bornens M. γ-Tubulin in mammalian cells: The centrosomal and the cytosolic forms. J Cell Sci. 1996. 109:875–887.
19. Park JH, Choi YL, Kwon DJ, Hwang IS, Park CK, Yang BK, Cheong HT. Control of MPF activity of recipient oocytes and subsequent development and DNA methylation of somatic cell nuclear transfer bovine embryos. Reprod Dev Biol. 2009. 33:223–228.
20. Raff JW, Kellogg DR, Alberts BM. Drosophila γ-tubulin is part of a complex containing two previously identified centrosomal MAPs. J Cell Biol. 1993. 121:823–835.

21. Rosenkrans CF Jr, First NL. Culture of bovine zygotes to the blastocyst stage: effects of amino acids and vitamins. Theriogenology. 1991. 35:266.

22. Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995. 7:39–45.

23. Shin MR, Park SW, Shim H, Kim NH. Nuclear and microtubule reorganization in nuclear-transferred bovine embryos. Mol Reprod Dev. 2002. 62:74–82.

24. Stearns T, Evans L, Kirschner M. γ-Tubulin is a highly conserved component of the centrosome. Cell. 1991. 65:825–836.

25. Tani T, Kato Y, Tsunoda Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biol Reprod. 2001. 64:324–330.

26. Tani T, Kato Y, Tsunoda Y. Reprogramming of bovine somatic cell nuclei is not directly regulated by maturation promoting factor or mitogen-activated protein kinase activity. Biol Reprod. 2003. 69:1890–1894.

27. Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998. 394:69–74.

28. Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, Wells DN, Young LE. Somatic cell nuclear transfer. Nature. 2002. 419:583–586.

29. Yin XJ, Tani T, Yonemura I, Kawakami M, Miyamoto K, Hasegawa R, Kato Y, Tsunoda Y. Production of cloned pigs from adult somatic cells by chemically assisted removal of maternal chromosomes. Biol Reprod. 2002. 67:442–446.

30. Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995. 378:578–583.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download