Abstract
Multidetector row computed tomography (MDCT) provides anatomical information about the kidney and other internal organs. Presently, the suitability of 64-channel MDCT to assess the kidney of healthy micropigs was evaluated. Morphological evaluations of the kidney and the major renal vessels of six healthy micropigs were carried out using MDCT, recording kidney volume and the diameter and length of renal arteries and veins. The mean diameters and lengths of the renal artery were 0.44 ± 0.05 and 4.51 ± 0.55 cm on the right side and 0.46 ± 0.06 and 3.36 ± 0.27 cm on the left side, respectively. The mean diameters and lengths of the renal vein were 1.44 ± 0.52 and 4.22 ± 1.29 cm on the right side and 1.38 ± 0.17 and 5.15 ± 0.87 cm on the left side, respectively. The mean volume of the right kidney was 79.3 ± 14.5 mL and of the left kidney was 78.0 ± 13.9 mL. The data presented in this study suggest that the MDCT offers a noninvasive, rapid, and accurate method for the evaluation of the renal anatomy in living kidney donors. It also provides sufficient information about extra-renal anatomy important for donor surgery and determination of organ suitability.
Transplantation is used to treat fulminate organ failure, but severe shortages in the availability of suitable human donors have limited the application of organ transplants [4]. This donor shortage has stimulated interest in the possible use of animal organs for transplantation into humans. Animal-to-human transplantation (xenotransplantation) would offer an unlimited supply of organs and tissue for transplantation. Pigs are the most likely source animals for xenotransplantation due to their anatomical and physiological similarities with humans [1]. Additionally, the pig can be raised to obtain large numbers of specific-pathogen-free animals. The reproductive properties of pigs such as early sexual maturity, short gestation time, and generation of large litters can allow a large pool of animal donors for xenotransplantation [18]. The ability to genetically modify the pig also allows modification of the targets of the human immune response and amelioration of some aspects of the rejection process without directly affecting the recipient's immune system.
In order to transplant swine organs into humans, physiological or anatomical comparison and analyses are essential in the investigation of whether an individual donor organ is suitable to a prospective patient. However, an appropriate method for estimating micropig organs has not been established. This study examined the feasibility of evaluating the kidney and its related major vessels using multidetector row computed tomography (MDCT) in micropigs. In recent years, major technological improvements have been achieved in CT. The most significant development has been the introduction of MDCT, which has brought about substantial improvements in spatial and particularly temporal resolution [3,6]. The present study examined the feasibility of MDCT on evaluation of kidney and renal vascular system of micropig as a potential renal donor.
All experimental protocols were approved by the Ethics Committee of Chonnam National University (CNU IACUCYB-2008-29). The study was conducted on six male Yucatan micropigs purchased from PWG Genetics Korea (Korea). The micropigs were physically healthy. They were housed in individual cages at the central animal facility and received a standard pig meal ad libitum. The mean age and body weight of the micropigs was 360 days and 30.50 ± 1.24 kg, respectively. Before the CT imaging procedures, all animals were fasted for a minimum of 24 h. Before MDCT image acquisition, the animals were premedicated using an intramuscular injection of azaperone (0.5 mg/kg) and xylazine (8 mg/kg), and anesthetized with an intramuscular injection of a zolazepam/tiletamine cocktail (4.4 mg/kg).
CT examination was performed on a 64-channel multidetector row helical CT scanner (Lightspeed VCT; GE Healthcare, USA) with following parameters: 0.5 sec per rotation, 5 mm collimation, pitch of 0.984 : 1, and tube current of 120 kV per 140~200 mAs. For administration of intravenous contrast material, a 20-gauge peripheral line was inserted in an ear vein. After a scout CT image was obtained, arterial phase volumetric image data sets were acquired 15 sec after the start of an intravenous injection of 60 mL of a nonionic contrast agent (Ultravist 370; Schering AG, Germany) at an injection rate of 3 mL/sec using an automated injector (LF CT 9000; Liebel-Flarsheim, USA). All image acquisitions were obtained in the craniocaudal direction and in the supine position. The imaging volume in arterial phase imaging extended from above the kidneys to just below the common iliac arteries. The volumetric data sets were then transferred to workstation (GE Advantage Workstation 4.3; GE Healthcare, USA) equipped with three-dimensional (3D) software (Volume Viewer Plus; GE Healthcare, USA) for subsequent review. Transverse 0.625 mm-thick sections were reformatted into maximum intensity projection images and volume rendered images.
One radiologist reviewed the CT images at the workstation. The reviewer used source images as well as 3D display images. For 3D CT angiography, volume-rendering techniques were usually used, but maximum-intensity-projection rendering was also used as an adjunct display. Renal arterial and venous anatomy was evaluated primarily on arterial phase images. The number and origin of renal arteries, the presence of early branching arteries, and any intrinsic renal artery disease was recorded. Renal venous anatomy was evaluated for the presence of accessory, retroaortic, or circumaortic veins. The reviewer recorded the number and diameter of renal arteries, and veins found on each side. Any branch within 2.0 cm from the aorta was classified as early branching. The diameter of the main renal artery and vein was assessed from the most appropriate point of the segment, 1 to 1.5 cm from the ostium. The length of renal vessels was assessed on coronal 3D CT angiographic images and defined as the distance from the ostium to the renal hilum. The kidney volume was measured from contiguous slices. In coronal reformatted images, the region of interest was drawn around the kidney, and the slices were reconstructed at 1-mm intervals to obtain a 3D volume-rendered image of the kidney. The volume was calculated by multiplying the sum of areas from each slice by the reconstruction interval at the workstation.
The results were presented as mean ± SD. The two-tailed t-test was used to compare values between the right and left kidneys. The results were considered to be significant when the p value was < 0.05. Statistical analysis was performed using software (SPSS version 15.0; SPSS, USA).
CT examinations were successfully performed in all animals. The mean body weight of the micropigs was 30.5 kg. There were 12 renal arteries in six micropigs. No accessory renal arteries or early branching arteries were detected. No arterial stenosis, aneurysm, or calcification was noted in any micropig (Fig. 1). The mean diameter of all 12 renal arteries was 0.45 ± 0.05 cm (min = 0.39, max = 0.56). The mean diameters of the renal artery were 0.44 ± 0.05 cm on the right side and 0.46 ± 0.06 cm on the left side. The mean lengths of the renal artery were 4.51 ± 0.55 cm on the right side and 3.36 ± 0.27 cm on the left side. The right renal artery was significantly longer than the left renal artery (p < 0.05).
There were 13 renal veins in the six animals. Two renal veins on the right side were observed in one micropig. No retroaortic or circumaortic renal vein was observed (Fig. 2). The mean diameter of the 13 renal veins was 1.41 ± 0.38 cm (min = 1.04, max = 2.51). The mean diameters of the renal vein were 1.44 ± 0.52 cm on the right side and 1.38 ± 0.17 cm on the left side. The mean lengths of the renal vein were 4.22 ± 1.29 cm on the right side and 5.15 ± 0.87 cm on the left side. The left renal vein was significantly longer than the right renal vein (p < 0.05).
The mean volume of all kidneys was 78.6 ± 13.6 mL (min = 61.6, max = 101.04). The mean volumes of the right kidney were 79.3 ± 14.5 mL and those of the left kidney were 78.0 ± 13.9 mL (Table 1). There was no significant difference in renal volume between the right and left kidneys. MDCT revealed no abnormalities in ureters and renal parenchyma in all micropigs. Furthermore, when compared with human data, considerable anatomic similarities were apparent between human and micropigs in renal vascular dimensions, although differences in renal volume were evident (Table 2).
Kidney transplants from living donors have become increasingly common during the past decade. Anatomical assessment of the donor kidneys is performed prior to transplantation to help select the kidney to be used and to plan the surgical approach [9]. An ideal imaging test should be free of morbidity, accurately estimate vascular and parenchymal structures, and recognize stone disease, renal parenchymal lesions, and other intra-abdominal pathologies. In human cases, preoperative knowledge of renal vascular, parenchymal, and urothelial anatomy based on imaging has always been important, not only to select suitable donor and kidney but also to avoid potential donor complications such as hemorrhage and potential recipient problems such as graft ischemia and urine leakage [14]. Although the micropig, a potential organ donor, should not be considered concerning postoperative complications, anatomical differences between donor and recipient should be determined. Indeed, congenital polycystic kidneys often occur in swine and can be inherited as an autosomal dominant trait [10]. Therefore, it is very important to establish the preoperative evaluation and selection system of the appropriate donor organ in micropigs.
In this study, we measured the vascular and volumetric parameters of right and left kidneys of micropigs using 64-channel MDCT. We reconfirmed that micropigs may be an appropriate renal donor because of the absence of arterial variations such as an accessory or early branching renal artery, and venous anomalies including retroaortic vein or circumaortic veins. In addition, comparison with human data revealed considerable anatomic similarities between human and micropigs in renal vascular dimensions, although differences in renal volume were found.
Since the introduction of MDCT in the early 1990s, the number of detectors has gradually increased [11], with 64-channel MDCT clinically used on humans at present. The advantages of MDCT include faster image acquisition, improved image resolution, and superior contrast reinforcement [2,19]. In addition, multiplanar reformation using MDCT is an optimal tool for imaging complicated anatomic and pathologic cases [7].
Conventional catheter digital subtraction angiography (DSA) has been performed on potential renal donors to show the number of renal arteries and the presence of early branching and vascular disease. However, it is invasive and sometimes traumatic, needs post-procedure observation, and has significant limitations in displaying renal veins and renal parenchyma. MDCT offers noninvasive imaging with minimal risk of morbidity. Over the past few years, MDCT has been used increasingly for noninvasive renal imaging [15,17]. MDCT angiography is highly accurate for detecting vascular anomalies and providing anatomic information of the kidney [8]. In addition, previous results showed that a more accurate evaluation of the renal arteries of potential donors can be achieved with MDCT angiography that with 3D magnetic resonance angiography. Furthermore, MDCT angiography can be used instead of digital subtraction angiography because the former can almost perfectly assess the number of renal arteries and the presence of proximal branches of the renal artery [9]. In addition to the vascularization of kidney, MDCT is usually used to detect various pathological conditions of the kidney including congenital anomalies, renal masses, cystic kidneys, or obstructive renal diseases [15]. Therefore, MDCT is an appropriate method is collecting data for the determination of normality and suitability of donor kidneys for transplantation.
In conclusion, 64-channel MDCT imaging allows the accurate determination of kidney morphological and spatial aspects, and understanding of renal vascular structures.
Figures and Tables
Fig. 1
(A) Posteroanterior views of the coronal maximum intensity projection image and (B) the volume-rendered image showing both the single right and left renal arteries (arrows). IVC: inferior vena cava; RK: right kidney; LK: left kidney; RV: right renal vein; LV: left renal vein.
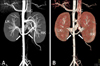
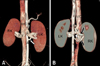
Fig. 2
(A) Anterior coronal volume-rendered image showing both the right and left renal veins. (B) Posteroanterior view of the coronal volume-rendered image showing two right renal veins (black arrows) that drain into the inferior vena cava separately. The white arrow indicates the left renal artery. The arrowhead indicates the right renal artery that runs between two right renal veins. A: abdominal aorta; IVC: inferior vena cava; RK: right kidney; LK: left kidney; RV: right renal vein; LV: left renal vein.
Acknowledgments
This work was supported by a grant (code #20070401034 006) from the BioGreen 21 Program, Rural Development Administration, Korea. The authors would like to acknowledge a graduate fellowship provided by the Ministry of Education and Human Resources Development through the Brain Korea 21 Project, Korea.
References
1. Dooldeniya MD, Warrens AN. Xenotransplantation: where are we today? J R Soc Med. 2003. 96:111–117.

2. Foley WD. Special focus session: multidetector CT: abdominal visceral imaging. Radiographics. 2002. 22:701–719.
3. Grude M, Juergens KU, Wichter T, Paul M, Fallenberg EM, Muller JG, Heindel W, Breithardt G, Fischbach R. Evaluation of global left ventricular myocardial function with electrocardiogram-gated multidetector computed tomography: comparison with magnetic resonance imaging. Invest Radiol. 2003. 38:653–661.

4. Hosenpud JD, Bennett LE, Keck BM, Fiol B, Boucek MM, Novick RJ. The registry of the International Society for Heart and Lung Transplantation: fifteenth official report-1998. J Heart Lung Transplant. 1998. 17:656–668.

5. Janschek EC, Rothe AU, Hölzenbein TJ, Langer F, Brugger PC, Pokorny H, Domenig CM, Rasoul-Rockenschaub S, Mühlbacher F. Anatomic basis of right renal vein extension for cadaveric kidney transplantation. Urology. 2004. 63:660–664.

6. Juergens KU, Maintz D, Grude M, Boese JM, Heimes B, Fallenberg EM, Heindel W, Fischbach R. Multi-detector row computed tomography of the heart: does a multi-segment reconstruction algorithm improve left ventricular volume measurements? Eur Radiol. 2005. 15:111–117.

7. Kalender WA, Polacin A. Physical performance characteristics of spiral CT scanning. Med Phys. 1991. 18:910–915.

8. Kawamoto S, Montgomery RA, Lawler LP, Horton KM, Fishman EK. Multidetector CT angiography for preoperative evaluation of living laparoscopic kidney donors. AJR Am J Roentgenol. 2003. 180:1633–1638.

9. Kim T, Murakami T, Takahashi S, Hori M, Takahara S, Ichimaru N, Okuyama A, Narumi Y, Nakamura H. Evaluation of renal arteries in living renal donors: comparison between MDCT angiography and gadolinium-enhanced 3D MR angiography. Radiat Med. 2006. 24:617–624.

10. Newman SJ, Confer AW, Panciera RJ. McGavin MD, Zachary JF, editors. Urinary system. Pathologic Basis of Veterinary Disease. 2006. 4th ed. St. Louis: Elsvier;613–691.
12. Saldarriaga B, Pérez AF, Ballesteros LE. A direct anatomical study of additional renal arteries in a Colombian mestizo population. Folia Morphol (Warsz). 2008. 67:129–134.
13. Shin HS, Chung BH, Lee SE, Kim WJ, Ha HI, Yang CW. Measurement of kidney volume with multi-detector computed tomography scanning in young Korean. Yonsei Med J. 2009. 50:262–265.

14. Smith PA, Ratner LE, Lynch FC, Corl FM, Fishman EK. Role of CT angiography in the preoperative evaluation for laparoscopic nephrectomy. Radiographics. 1998. 18:589–601.

16. Turba UC, Uflacker R, Bozlar U, Hagspiel KD. Normal renal arterial anatomy assessed by multidetector CT angiography: are there differences between men and women? Clin Anat. 2009. 22:236–242.

17. Valastro M, Veroux M, Macarone M, Cappello D, Vizcarra D, Gagliano M, Di Mare M, Spataro M, Giuffrida G, Tallarita T, Magnano San Lio V, Veroux P. Multi-detector row CT scanner angiography in the evaluation of living kidney donors. Chir Ital. 2007. 59:337–341.
18. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997. 385:810–813.

19. Wintersperger BJ, Herzog P, Jakobs T, Reiser MF, Becker CR. Initial experience with the clinical use of a 16 detector row CT system. Crit Rev Comput Tomogr. 2002. 43:283–316.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download