Abstract
In order to evaluate the genetic variability of the causative agent of cold water disease (CWD), plasmid profiling was used to characterize Flavobacterium (F.) psychrophilum isolates (n = 169). Size analysis of plasmids in F. psychrophilum isolates (n = 128) from several fish species demonstrated that six kinds of plasmids were harbored, and ayu isolates had different profiles compared to other isolates. Moreover, multiple isolates (n = 41) from CWD outbreaks in 2002 to 2003 at a single ayu farm were examined to determine differences between isolates from successive outbreaks and showed different profiles by the sources of seedlings.
In Japan, the causative agent of cold water disease (CWD), Flavobacterium (F.) psychrophilum, was first isolated from ayu (Plecoglossus altivelis altivelis) in 1987 [8] and spread widely over many host fish species in various areas, causing severe losses in aquaculture [4]. Recently, different typing methods including serotyping [9], genotyping [4] and plasmid profiling [2,3,5,7] have been evaluated for the comparison of F. psychrophilum isolated from ayu, and these studies revealed that isolates with a specific serotype [9] or genotype [4] are specifically infectious to ayu and scarcely infectious to other fish species. However, these studies did not reflect the epidemiological situation of CWD in Japan. Therefore, the aim of this study was to determine the plasmid profiles to assist in characterizing Japanese F. psychrophilum isolates from several fish species and CWD outbreaks in one ayu farm which cultured different sources of seedlings.
A total of 169 F. psychrophilum isolates were used in this study. Among the isolates, 128 were provided either by prefecture disease control centers or fishery research stations, and 41 were collected from a single ayu farm (B farm) in Japan. All the isolates were stored in cytophaga broth (CB) [1] with 10% glycerol at -80℃ and subcultured in CB at 18℃ for 48 h. For bacterial plasmid DNA extraction, bacterial cell pellets of 169 isolates grown in CB were harvested after 3 min of centrifugation at 10,000 × g. The pellets were washed twice with DW. For plasmid DNA preparation, QIAprep Miniprep Kit (Qiagen GmbH, Germany) was used. The plasmids were separated on 0.70% agarose ME-TAE gels along with length markers (λ DNA-Hind III digest; New England BioLabs, USA) via electrophoresis (100 V, 60 min) and analyzed (Fig. 1).
In the first experiment, the plasmid profiles from 128 isolates and their relationship with host fish were analyzed. Eighty-three of 128 isolates harbored four kinds of single plasmids (1.8 kb, 2.4 kb, 2.7 kb or 3.5 kb) and ten of the eighty-three isolates harbored combinations of two different types of plasmids from ayu (3.5 kb and 2.4 kb or 3.5 and 1.8 kb), pale chub Zacco platypus (100 kb and 23 kb) and crucian carp Carassius cuvieri (100 kb and 23 kb; 3.5 kb and 2.4 kb) (Table 1). The single plasmid results were similar to previous studies [2,5], except for the new types of plasmids (1.8 kb, 23 kb and 100 kb). These results showed different plasmid profiles among host fish species compared to the previous studies [2,3,5,7]. Additionally, several types of plasmids (3.5 kb, 2.4 kb and 1.8 kb) were found mostly in ayu compared to other host fish species.
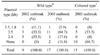
The similar plasmid harboring patterns were also observed in the second experiment, which examined the 41 isolates from diseased ayu at B Farm and experienced CWD outbreaks in successive years (2002 and 2003). Twenty-six strains were from diseased wild ayu grown from seedlings from Lake Biwa in 2002 and 2003 (wild type) and 15 were from diseased cultured ayu grown from seedlings from the Tokushima Prefecture Fish Farming Center (Japan) in 2003 (cultured type). Twenty-nine of 41 isolates harbored three types of plasmids (3.5 kb and 1.8 kb, 3.5 kb, 2.4 kb) similar to the plasmid sizes and profiles to the first experiment. The wild type ayu isolates harbored either single or a combination of two different types of plasmids, while the cultured showed only a single type of plasmid. Moreover, F. psychrophilum isolated in the wild type predominantly harbored 2.4 kb (55.5%) in 2002 and 3.5 kb (64.7%) in 2003, showing annual differences. In contrast, F. psychrophilum isolated in cultured types predominantly harbored no plasmid (66.7%) in 2002 and 3.5 kb (33.3%) plasmid in 2003 (Table 2). These findings may demonstrate that F. psychrophilum exhibiting special plasmid profiles (3.5 kb and 1.8 kb, 3.5 kb, 2.4 kb) are specifically infectious to ayu, and the cultured ayu have limited exposure to F. psychrophilum harboring different types of plasmids compared to wild type even when isolated in the same fish farm. It was previously described that F. psychrophilum with different serotypes and ribotypes were isolated from the same salmonid farm [6]. It also explains that F. psychrophilum may transmit its plasmid because of unlimited exposure to other isolates in the wild environment. Therefore, it can be speculated that the specific size of plasmids is related to its host fish species, and more than one strain harboring different kinds of plasmids can simultaneously infect a single farm.
In conclusion, the differentiation of F. psychrophilum is particularly important for the tracking of disease outbreak sources and for understanding the epidemiological aspects of CWD. Additionally, in order to ensure effective CWD vaccination, bacterial variations must be fully documented. Based on our study, it was demonstrated that F. psychrophilum exhibiting special plasmid profiles are specifically infectious to ayu and F. psychrophilum isolated from wild ayu may differ from cultured ayu even when isolated from the same farm displaying annual variations in plasmid profiles. Although the functions of F. psychrophilum plasmids still remain unknown, these results may provide a basis for understanding the epidemiological situation of CWD in Japanese ayu farms.
Figures and Tables
Fig. 1
Plasmids profiles extracted from Flavobacterium psychrophilum isolates using 0.70% agarose ME-TAE gels. Lane M; marker (λ DNA-Hind III digest), Lane 1; 3.5 and 2.4 kb (ayu isolate), Lane 2; 3.5 and 2.4 kb (crucian carp isolate), Lane 3; 3.5 and 1.8 kb (ayu isolate), Lane 4; 3.5 kb (ayu isolate), Lane 5; 2.7 kb (rainbow trout isolate), Lane 6; 2.4 kb (ayu isolate), Lane 7; 1.8 kb (ayu isolate), Lane 8; 100 and 23 kb (crucian carp isolate), Lane 9; none (ayu isolate).

Acknowledgments
We would like to thank the staffs of Tokushima and Hiroshima Prefectural Fisheries Experiment Stations. This study was financially supported by a Korea Research Foundation Grant (KRF-2008-331-E00385).
References
1. Anacker RL, Ordal EJ. Studies on the myxobacterium Chondrococcus columnaris. I. Serological typing. J Bacteriol. 1959. 78:25–32.

2. Chakroun C, Grimont F, Urdaci MC, Bernardet JF. Fingerprinting of Flavobacterium psychrophilum isolates by ribotyping and plasmid profiling. Dis Aquat Organ. 1998. 33:167–177.

3. Izumi S, Aranishi F. Plasmid profiling of Japanese Flavobacterium psychrophilum isolates. J Aquat Anim Health. 2004. 16:99–103.
4. Izumi S, Aranishi F, Wakabayashi H. Genotyping of Flavobacterium psychrophilum using PCR-RFLP analysis. Dis Aquat Organ. 2003. 56:207–214.
5. Lorenzen E, Dalsgaard I, Bernardet JF. Characterization of isolates of Flavobacterium psychrophilum associated with coldwater disease or rainbow trout fry syndrome I: phenotypic and genomic studies. Dis Aquat Organ. 1997. 31:197–208.

6. Madetoja J, Hänninen ML, Hirvelä-Koski V, Dalsgaard I, Wiklund T. Phenotypic and genotypic characterization of Flavobacterium psychrophilum from Finnish fish farms. J Fish Dis. 2001. 24:469–479.

7. Madsen L, Dalsgaard I. Comparative studies of Danish Flavobacterium psychrophilum isolates: ribotypes, plasmid profiles, serotypes and virulence. J Fish Dis. 2000. 23:211–218.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download