Abstract
Avian metapneumovirus (aMPV) causes upper respiratory tract infections in chickens and turkeys. Although the swollen head syndrome (SHS) associated with aMPV in chickens has been reported in Korea since 1992, this is the study isolating aMPV from chickens in this country. We examined 780 oropharyngeal swab or nasal turbinate samples collected from 130 chicken flocks to investigate the prevalence of aMPV and to isolate aMPV from chickens from 2004-2008. Twelve aMPV subtype A and 13 subtype B strains were detected from clinical samples by the aMPV subtype A and B multiplex real-time reverse transcription polymerase chain reaction (RRT-PCR). Partial sequence analysis of the G glycoprotein gene confirmed that the detected aMPVs belonged to subtypes A and B. Two aMPVs subtype A out of the 25 detected aMPVs were isolated by Vero cell passage. In animal experiments with an aMPV isolate, viral RNA was detected in nasal discharge, although no clinical signs of SHS were observed in chickens. In contrast to chickens, turkeys showed severe nasal discharge and a relatively higher titer of viral excretion than chickens. Here, we reveal the co-circulation of aMPV subtypes A and B, and isolate aMPVs from chicken flocks in Korea.
Avian metapneumovirus (aMPV) is a member of the subfamily Pneumovirinae in the family Paramyxoviridae [26]. aMPV, also known as turkey rhinotracheitis virus, causes a widespread disease of turkeys, chickens and some other avian species [7]. The disease is characterized by an upper respiratory tract infection in turkeys. aMPV is also considered to be a predisposing factor for triggering swollen head syndrome (SHS) in broiler and broilers breeders [11, 13], and egg production losses in layers [29]. In chickens, it is well-known that the complication of secondary bacterial infections with organisms like Escherichia coli [10] and the immunosuppressive effect of infectious bursal disease [24] are important in the development of typical SHS.
Since aMPV was first described in South Africa in 1978 [3], the virus has been reported in other parts of Europe and the Middle East, and is now considered to be a major disease threat in both turkeys and chickens in many parts of the world [1,2,5,23,27]. Several subtypes of aMPV have been recognized and differentiated by nucleotide sequence analysis based on the attachment (G) protein [14]. Subtype A was first isolated in South Africa and England, whereas subtype B virus was initially isolated in continental European countries including Hungary, Spain and Italy. Subtype C was isolated in the United States [27], while the presence of an additional subtype D was reported in France [2].
In Korea, SHS was first observed in 1992 [16] and antibodies to aMPV have been detected in 21 out of 30 broiler breeder flocks (70%) [21]. Although clinical diseases similar to SHS have continuously reported in chicken flocks by field veterinarians, there have been no reports isolating and identifying aMPV subtype A in Korea. Recently, aMPV subtype C was isolated from pheasants in Korea [20].
To investigate the prevalence of aMPV in chickens in Korea, we developed a multiplex real-time reverse transcription polymerase chain reaction (RRT-PCR) for rapid detection of aMPV subtypes A and B. Although various methods such as a RT-nested PCR assay have been used to detect aMPV [7], it is time-consuming and sometimes produces false positive results caused by DNA contamination during secondary PCR. Here, we examined oropharyngeal swab and nasal turbinate samples taken from aMPV monitoring chicken farms using multiplex RRT-PCR and tried to isolate aMPV by Vero cell passages. For further analysis of the molecular and pathogenic characteristics of subtype A aMPVs isolated in this study, we analyzed the phylogenetic trees and investigated the pathogenicity of a Korean aMPV isolate in chickens and turkeys.
Clinical samples were received for diagnosis and surveillance for aMPV during 2004-2008. A total of 130 chicken flocks (26 broiler, 87 broiler breeder and 17 layer flocks) with or without clinical signs ranging from mild to severe were examined. The oropharynx of six birds was swabbed per flock and each of two samples was pooled for this study. Oropharyngeal swabs or nasal turbinates were resuspended in minimum essential medium with gentamicin (Kukjae Pharmaco, Korea), the suspension was clarified by centrifugation at 1,000 × g for 10 min, and the supernatant was used immediately for RNA extraction and aMPV isolation.
aMPV subtype A (Nobilis TRT vaccine; Intervet, The Netherlands) and subtype B (Nemovac; Merial, USA) vaccine strains were used as reference for both multiplex RRT-PCR and RT-PCR. Vero cells were prepared for aMPV isolation.
RNA was extracted directly from oropharyngeal swab or nasal turbinate samples using an RNeasy kit (Qiagen, USA) according to the manufacturer's instructions. To detect aMPV in samples, multiplex RRT-PCR was developed with primers and probes designed for this study (Table 1). QuantiTect Probe RT-PCR kit (Qiagen, USA) was used for the multiplex RRT-PCR reaction. Each reaction consisted of 10 µL total RNA, 12.5 µL QuantiTect master mix, 0.25 µL QuantiTect RT mix, 0.375 µL forward and reverse primers (each 55 µM) and 0.375 µL probes (each 14 µM), for a final volume of 25 µL. The multiplex RRT-PCR was performed on a SmartCycler (Cepheid, USA) with the following conditions: one cycle at 50℃ for 30 min, 95℃ for 15 min followed by 45 cycles of 94℃ for 15 sec, 55℃ for 60 sec. For the sequencing, RT-PCR was performed with primers as described by Cavanagh et al. [4]. The RT-PCR products were amplified for 268 base pair (bp) or 361 bp fragments corresponding to subtypes A and B, respectively, of the aMPV G gene.
Oropharyngeal swab or nasal turbinate samples confirmed to be aMPV positive by RRT-PCR were directly inoculated and passaged in Vero cells until cytopathic effects (CPE) were evident. When the Vero cells showed CPE, the aMPV was identified by immunofluorescence assay with the monoclonal antibody TRT12 (provided by National Veterinary Research and Quarantine Service, Korea) and confirmed by transmission electron microscopy.
To analyze the G glycoprotein from aMPV-positive samples, RT-PCR products (268 bp and 361 bp) were purified with the QIAquick PCR purification kit (Qiagen, USA) and sequenced by automated DNA sequencing using an ABI 377 apparatus (Applied Biosystems, USA). Sequences were aligned with the aMPV vaccine strains (Nobilis TRT vac/A and Nemovac/B) and previously published sequences: CVL14/1 (accession number L34032) and 8544 from England (DQ666911), 1556 from France (L34030), LAH (AY640317), SHSBR662/03 (AY739717), SHSBR668/03 (AY739718), SHSBR669/03 (AY739719), TRTBR169 (AY734531), chicken/A/BR/119/95 (AY842242), chicken/A/BR/121/95 (AY842243), aMPV/ B/Brazil-07/ USP-06 (EU140747), aMPV/B/Brazil-06/USP-07 (EU140748) and aMPV/B/Brazil-07/ USP-08 (EU140749) from Brazil, 872S from Spain (L34034), 6574 from Hungary (L34033), 2119 from Italy (L34031), Isr/1708/02 from Israeli (AY728268), Colorado 2 from United States (AY590688), PL-1 (EF199771) and PL-2 (EF199772) from Korea. The multiple sequence alignments were prepared with ClustalW and phylogenies were reconstructed with MEGA 4.1 software [17] using the Neighbor-joining tree inference method, with 1,000 bootstrap replicates to assign confidence levels to branches.
The pathogenic response of chemically immunosuppressed chickens to aMPV was studied. Thirty-nine 3 week-old specific pathogen free (SPF) chickens were divided into three groups of 13. Each group was housed in separated positive pressure isolators. Chickens in group 1 were inoculated intramuscularly with 100 mg/kg of CsA (Cypol; Chongkundang, Korea) per chicken, every 3 days from 3 weeks of age until the end of the experiment as previously described [18], and chickens in group 2 were not treated with CsA. At 31 days of age, both groups were inoculated oculonasally with 104 TCID50/0.1 mL of aMPV Ck/A/Kr/1336/06. Chickens in group 3 were kept as the untreated and unchallenged control. Nasal discharge and oropharyngeal swabs were collected from each group at 2, 3, 4, 5, 6 and 7 days post challenge (dpc). The virus titer of each specimen was determined using quantitative RRT-PCR. Standard curves were generated with control viral RNAs and the cycle threshold values of samples were converted into TCID50/mL by interpolation as previously described [19,28]. Three chickens from each group were sacrificed at 5 dpc and the turbinate, trachea and lungs collected for histopathological studies. Ten chickens of each group were monitored daily for 14 days for any clinical signs. Blood was collected from all remaining chickens and serum was tested for the presence of aMPV antibodies by enzyme-linked immunosorbant assay (ELISA). All experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee of Konkuk University.
Twenty-six 2 week-old turkeys were divided into two groups of 13. Each group was housed in separated positive pressure isolators. Turkeys in group 1 were inoculated oculonasally with 104 TCID50/0.1 mL of aMPV Ck/A/Kr/1336/06, while group 2 was the unchallenged control. Nasal discharge and oropharyngeal swabs were collected at 1, 3, 5, 7 and 9 dpc and tested for the shedding titer of aMPV by multiplex RRT-PCR. Three turkeys from each group were sacrificed at 5 dpc and the turbinate, trachea and lungs collected for histopathological studies. Ten turkeys of each group were monitored daily for 14 days for any clinical signs and blood collected to test for aMPV antibodies.
Nasal turbinate, trachea and lungs were collected from 3 chickens and turkeys at 5 dpc, formalin-fixed and processed. Paraffin sections were stained with hematoxylin and eosin using standard procedures for microscopic examination.
Sera were tested for the presence of aMPV antibodies using a commercial aMPV ELISA kit, avian rhinotracheitis antibody test kit (BioCheck, The Netherlands) according to the manufacturer's instructions. Based on the optical densities of the samples (405 nm), sample to positive (S/P) ratios were calculated and used to express the mean S/P ratio per group. Samples with an S/P of 0.5 or greater were considered to contain anti-aMPV antibodies, and were judged positive based on the manufacturer's recommendations. S/P of 0.35~0.499 was considered suspect for aMPV antibodies and a ratio of 0.349 or less was considered to be negative.
As shown in Table 2, four of the 156 samples from broiler chickens (2.6%), twenty of the 522 samples from broiler breeders (3.8%), and one of 102 samples from layers (1.0%) were detected by multiplex RRT-PCR. One subtype A and three subtype B aMPVs were detected from broiler flocks and 10 subtype A and 10 subtype B aMPVs were detected in broiler breeder flocks. One A subtype aMPV was detected in layer flocks.
Either 268 bp (subtype A) or 361 bp (subtype B) fragments of the G gene determined from all aMPV positive samples by multiplex RRT-PCR were amplified by RT-PCR for sequence analysis. A yield of seven partial sequences of the G gene was the result. The nucleotide sequences of the G gene of aMPV subtypes A and B received the following Genbank accession numbers: Ck/A/Kr/567/04 (FJ796704), Ck/A/Kr/166/04 (FJ796707), Ck/A/Kr/167/04 (FJ796706), Ck/Kr/A/1336/06 (FJ796703), Ck/A/Kr/301/08 (FJ796705), Ck/B/Kr/168/04 (FJ796702) and Ck/B/Kr/746/04 (FJ796701). Five genes clustered with subtype A reference strains and two genes clustered with subtype B reference strains (Fig. 1).
To isolate aMPV from field samples, IBV, NDV, ILTV and Mycoplasma gallisepticum were also screened. Samples that were positive for any of these other pathogens were not used for aMPV isolation, and the samples that were RRT-PCR positive for aMPV were candidates for virus isolation. The infected cell layer of each passage was trypsinized and reseeded into culture flasks. CPE was characterized by many rounded and floating cells, and appeared in Vero cells at 3~4 passages. Unfortunately, 23 aMPV positive samples by multiplex RRT-PCR disappeared upon passaging, and just two subtype A aMPVs (Ck/Kr/A/1336/06 and Ck/A/Kr/301/08) were isolated in Vero cells (Table 2). Ck/Kr/A/1336/06 was isolated from a broiler breeder and Ck/A/Kr/301/08 was isolated from a layer. When examined by negative stain electron microscopy, Ck/A/Kr/1336/06 appeared as long filamentous forms (Fig. 2). In conjunction with electron microscopy, the isolate was confirmed by immunofluorescence assay using TRT12 monoclonal antibody (data not shown). CK/Kr/A/1336/06 isolated by passaging 4 times was directly used for pathogenicity testing in chickens and turkeys.
Because SHS cannot be induced by aMPV alone in chickens [10], SPF chickens were immunosupressed by CsA followed by inoculation with Korean aMPV to reproduce the disease. Both CsA-treated and non-treated chickens showed only nasal discharge at 3 dpc and did not show any histopathological lesions. None of the non-challenged chickens showed clinical signs or histological lesions. Viral replication was detected in the nasal discharge of the infected birds by RRT-PCR. As shown in Fig. 3, there were no differences in viral load between CsA-treated and non-treated chickens at 2, 3 and 4 dpc. However, at 5 (p < 0.05), 6 (p < 0.05) and 7 (p < 0.005) dpc, CsA-treated chickens excreted viruses at a higher titer than non-treated chickens. Although virus shedding from nasal discharge had ceased by 7 days post infection in non-treated chickens, CsA-treated chickens continuously shed the virus until 7 days post infection.
To determine the pathogenicity of Korean aMPV in commercial turkeys, 10 two-week-old turkeys were inoculated with Korean aMPV. Four of the 10 turkeys began to show nasal discharge at 3 dpc, and seven of the 10 turkeys showed symptoms such as nasal discharge, sinus swelling and watery eyes at 5 dpc (data not shown). None of the non-challenged turkeys showed clinical signs or histological lesions. The presence of clinical signs in aMPV infected turkeys was associated with histological lesions characterized by mild to severe inflammation in the turbinate and trachea (Fig. 4). As shown in Fig. 3, significantly higher virus titer was shed from nasal discharge than oropharyngeal swabs at 3 (p < 0.05), 5 (p < 0.05), and 7 (p < 0.005) dpc, and aMPV infected turkeys shed the virus from both oropharyngeal swab and nasal discharge until 9 dpc.
On the day of aMPV challenge, all sera collected from birds confirmed that chickens and turkeys were negative for aMPV (Table 3). In experiment 1, at 14 dpc, three of 10 sera of CsA-treated SPF chickens were positive for aMPV antibodies (mean S/P ratio = 0.58 ± 0.55). In CsA non- treated chickens, two of 10 sera were suspect for aMPV antibodies. No antibodies against aMPV were detected in serum samples of CsA non-treated and non-challenged chickens (0.10 ± 0.06). In experiment 2, seven of 10 sera of turkeys were positive, showing high antibody levels (mean S/P ratio = 1.03 ± 0.85) and no antibodies against aMPV were detected in serum samples of non-challenged turkeys (0.10 ± 0.01) at 14 dpc.
Although SHS has been reported in Korea since 1992 [16], this is the first study isolating and characterizing aMPV subtype A from chickens in Korea. In this study, we isolated two subtype A aMPV strains from chickens. One was isolated from a broiler breeder flock showing facial swelling especially around the eye, nasal discharge, egg production losses and discolored eggs. The other subtype was isolated from a layer flock in which egg production losses were evident (data not shown). According to necropsy results of broiler breeders, severe nasal discharge induced blockage of the larynx of the chickens, delaying the feeding time, resulting in a fatty liver and, finally, lower productivity. In most reports of aMPV infection in chickens, the focus is on SHS associated with aMPV. In this study, however, we assumed that severe nasal discharge induced by aMPV caused delayed feeding time and a lower productivity, as well as SHS linked with aMPV.
Due to the poor replication of aMPV in chickens [8,12], isolation of aMPV from chickens is limited. Furthermore, because clinical signs induced by aMPV alone are not apparent in chickens, virus isolation from chickens is more difficult than from turkeys [12]. We determined that three factors are important in the isolation of aMPV from chickens. The first one is sampling at the early stage of the aMPV infection. Sampling in the early stage of the infection is crucial, as the virus may be present only in the nasal sinuses and turbinates for a short period when the chicken showing the very first symptoms of the disease [7]. The second factor concerns the type of sample. We attempted to isolate the aMPV from the nasal turbinate of chickens that did not show clinical signs in broiler breeder flock showing SHS, and ascertained that the detection rate of aMPV was higher in nasal turbinates than in oropharyngeal swabs (data not shown). The third important factor concerns the isolation method; reseeding of the aMPV by passage. Lu et al. [22] isolated the aMPV strain LU using tracheal organ culture and adapted aMPV to Vero cells passaged by trypsinizing and reseeding into culture flasks. In a slight modification to isolate aMPV from field samples, we directly inoculated these samples into Vero cells and passaged by trypsinizing and reseeding into culture flasks. Because of the poor replication of aMPV in the host [8,12], aMPV titer may be very low in the early passages of Vero cells. Therefore, it appears to be very critical that host cells be passaged by trypsinizing and reseeding into culture flasks.
As reported in Japan and Taiwan [22,23], and due to our geographic location, we expected to diagnose aMPV subtypes A and/or B at a high rate. Indeed, the survey results of aMPV from chicken flocks showed aMPV infection with co-circulation of subtypes A and B. Sequence analysis of parts of the attachment protein G gene showed a relative homogeneity within the Korean aMPV genes and confirmed that the aMPV detected samples belonged to subtypes A and B.
In animal experiments, although we induced immunosuppression in chickens to reproduce SHS, CsA treatment of chickens could not reproduce SHS. A previous study also unsuccessfully attempted to induce re-excretion of aMPV in poults and chicks using CsA immunosuppression [15]. The failure might reflect a situation where reproduction of SHS is not under T-cell control. However, considering the antibody responses in CsA-treated chickens in this study, it seems that T-cell suppression rendered the chickens more susceptible to infection by a aMPV isolate than non- CsA treated chickens. Although aMPV shedding titer from the nasal discharge of chickens and turkeys was similar, turkeys only showed clinical signs and histopathological lesions. These data demonstrated that the Korean aMPV isolate also causes severe respiratory infection in turkeys than chickens, as previously reported [6]. In contrast to the turkeys, aMPV infected chickens showed only transparent nasal discharge in spite of CsA treatment. Rhinovirus causes common cold accompanied with nasal discharge without the damage of nasal epithelium in humans. And nasal obstruction can interfere with sleep and feeding [30]. Despite the presence of mild clinical signs and virus shedding, histological lesions were not observed in affected chickens. Although further studies are essential, the pathogenesis of aMPV in chickens may be similar to rhinovirus infection in humans. As rhinovirus infected humans have disruptions in feeding, we could also check the delayed feeding time in aMPV infected broiler breeders caused by obstruction of the larynx. Consistent with a previous report [25], aMPV infected chickens also showed very low serum antibody levels. Because the aMPV replicate in upper respiratory tissues for only a short time [9], the low multiplication levels of aMPV in target tissues of chickens results in low antibody responses. These results could explain why the re-infection of aMPV occurs frequently in chicken flocks.
In summary, we confirmed aMPV infection in chickens and isolated aMPV from chickens in Korea. aMPV isolation from chickens showing severe nasal discharge suggests that it induces lower productivity due to upper respiratory passage blockage in chicken flocks. These results suggest aMPV infection in chicken flocks gives rise to appreciable economic losses in the poultry industry. Therefore, continuing epidemiological surveys are necessary for the prevention of aMPV infections in chicken flocks in Korea.
Figures and Tables
Fig. 1
Pylogenetic trees of avian metapneumovirus (aMPV). Nucleotides 161-383 (223 bases) of the G gene were used for phylogenetic analysis.

Fig. 2
Electron micrograph of the Korean aMPV isolate Ck/A/Kr/1336/06 derived from infected Vero cell culture.

Fig. 3
Viral shedding from nasal discharge of immunosuppressed specific pathogen free chickens in experiment 1 (A) and from oropharyngeal (OP) swabs and nasal discharge of turkeys in experiment 2 (B). The error bars indicate standard deviations from virus shedding titers of eight chickens per group. *p < 0.05. **p < 0.005.

Fig. 4
Histological lesions in the upper trachea (A, B) and nasal turbinate (C, D) of turkeys 5 days post challenge. (A) Upper trachea of the Korean aMPV isolate-inoculated turkey showing infiltration of lymphoid cells in the epithelium (black arrow). (B) Intact ciliated epithelium of a non-challenged turkey. (C) Nasal turbinate of the Korean aMPV isolate-inoculated turkey showing infiltration of lymphoid cells in the epithelium (black arrow) and hypertrophy of the mucous gland (white arrow). (D) Nasal turbinate of a non-challenged turkey. H&E stain, ×200.
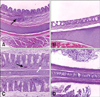
Table 3
Results of aMPV ELISA in experimentally challenged SPF chickens and turkeys

*S/P ratio: mean of test samples-mean of negative control/mean of positive control-mean of negative control. S/P ratio of 0.349 or less, 0.35~0.499 and 0.5 or greater are considered negative, suspect and positive, respectively. dpc: days post challenge. †Number of positive (number of suspect) for aMPV antibodies/number of tested.
Acknowledgments
The authors would like to thank Merial for supplying aMPV susceptible Vero cells and Samhwa farms for coordination of aMPV specimen collection. We also thank the National Veterinary Research and Quarantine Service for assistance in transmission electron microscopy.
References
1. Banet-Noach C, Simanov L, Perk S. Characterization of Israeli avian metapneumovirus strains in turkeys and chickens. Avian Pathol. 2005. 34:220–226.

2. Bäyon-Auboyer MH, Arnauld C, Toquin D, Eterradossi N. Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subgroup. J Gen Virol. 2000. 81:2723–2733.

3. Buys SB, Du Preez JH. A preliminary report on the isolation of a virus causing sinusitis in turkeys in South Africa and attempts to attenuate the virus. Turkeys. 1980. 28:36–46.
4. Cavanagh D, Mawditt K, Britton P, Naylor CJ. Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions. Avian Pathol. 1999. 28:593–605.

5. Collins MS, Gough RE, Alexander DJ. Antigenic differentiation of avian pneumovirus isolates using polyclonal antisera and mouse monoclonal antibodies. Avian Pathol. 1993. 22:469–479.

7. Cook JKA, Cavanagh D. Detection and differentiation of avian pneumoviruses (metapneumoviruses). Avian Pathol. 2002. 31:117–132.

8. Cook JKA, Ellis MM, Huggins MB. The pathogenesis of turkey rhinotracheitis virus in turkey poults inoculated with the virus alone or together with two strains of bacteria. Avian Pathol. 1991. 20:155–166.

9. Cook JKA, Kinloch S, Ellis MM. In vitro and in vivo studies in chickens and turkeys on strains of turkey rhinotracheitis virus isolated from the two species. Avian Pathol. 1993. 22:157–170.

10. Droual R, Woolcock PR. Swollen head syndrome associated with E. coli and infectious bronchitis virus in the Central Valley of California. Avian Pathol. 1994. 23:733–742.

11. Gough RE, Manvell RJ, Drury SE, Pearson DB. Isolation of an avian pneumovirus from broiler chickens. Vet Rec. 1994. 134:353–354.

12. Jones RC. Avian pneumovirus infection: questions still unanswered. Avian Pathol. 1996. 25:639–648.

13. Jones RC, Naylor CJ, Bradbury JM, Savage CE, Worthington K, Williams RA. Isolation of a turkey rhinotracheitis-like virus from broiler breeder chickens in England. Vet Rec. 1991. 129:509–510.
14. Juhasz K, Easton AJ. Extensive sequence variation in the attachment (G) protein gene of avian pneumovirus: evidence for two distinct subgroups. J Gen Virol. 1994. 75:2873–2880.

15. Khehra RS, Jones RC. Investigation into avian pneumovirus persistence in poults and chicks using cyclosporin A immunosuppression. Res Vet Sci. 1999. 66:161–163.

16. Kim JH, Song CS, Seong HW, Mo IP, Kwon JH, Kim KS, Kim SH. The investigation of sero-prevalence and occurrence of SHS from broiler breeder in Korea. Korean J Vet Res. 1992. 32:25.
17. Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004. 5:150–163.

18. Kwon JS, Lee HJ, Lee DH, Lee YJ, Mo IP, Nahm SS, Kim MJ, Lee JB, Park SY, Choi IS, Song CS. Immune responses and pathogenesis in immunocompromised chickens in response to infection with the H9N2 low pathogenic avian influenza virus. Virus Res. 2008. 133:187–194.

19. Lee CW, Suarez DL. Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J Virol Methods. 2004. 119:151–158.

20. Lee E, Song MS, Shin JY, Lee YM, Kim CJ, Lee YS, Kim H, Choi YK. Genetic characterization of avian metapneumovirus subtype C isolated from pheasants in a live bird market. Virus Res. 2007. 128:18–25.

21. Lee Y. Studies on detection of turkey rhinotracheitis virus using monoclonal antibody and polymerase chain reaction. 1995. Seoul: Konkuk University;27–29. Master's thesis.
22. Lu YS, Shien YS, Tsai HJ, Tseng CS, Lee SH, Lin DF. Swollen head syndrome in Taiwan-isolation of an avian pneumovirus and serological survey. Avian Pathol. 1994. 23:169–174.

23. Mase M, Yamaguchi S, Tsukamoto K, Imada T, Imai K, Nakamura K. Presence of avian pneumovirus subtypes A and B in Japan. Avian Dis. 2003. 47:481–484.

25. Otsuki K, Hirai N, Mitani M, Itani M, Shimohata T, Kunii E, Uramoto K, Kiyotake M, Kato H, Ellis MM, Cook JK. Demonstration of serum-neutralising antibody to turkey rhinotracheitis virus in serum from chicken flocks in Japan. J Vet Med Sci. 1996. 58:869–874.

27. Seal BS. Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains. Virus Res. 1998. 58:45–52.

28. Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002. 40:3256–3260.

29. Sugiyama M, Koimaru H, Shiba M, Ono E, Nagata T, Ito T. Drop of egg production in chickens by experimental infection with an avian metapneumovirus strain PLE8T1 derived from swollen head syndrome and the application to evaluate vaccine. J Vet Med Sci. 2006. 68:783–787.

30. Van Kempen M, Bachert C, Van Cauwenberge P. An update on the pathophysiology of rhinovirus upper respiratory tract infections. Rhinology. 1999. 37:97–103.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download