Abstract
An 8-year-old male Austrian Pinscher and a 14-year-old male Golden Retriever were presented for evaluation due to unexplainable high fructosamine values despite euglycemia and epistaxis in combination with polydipsia/polyuria, respectively. Blood analysis revealed severe hyperglobulinemia, hypoalbuminemia and markedly elevated fructosamine concentrations in both dogs. Multiple myeloma with IgA-monoclonal gammopathy was diagnosed by serum and urine electrophoresis including immunodetection with an anti-dog IgA antibody and bone marrow aspirations. Diabetes mellitus was excluded by repeated plasma and urine glucose measurements. Fructosamine values were positively correlated with globulin, but negatively correlated with albumin concentrations. These cases suggest that, as in human patients, monoclonal IgA gammopathy should be considered as a possible differential diagnosis for dogs with high fructosamine concentrations.
Fructosamines are glycated proteins that are measured to identify diabetes mellitus and as indicators of glycemic control [4,9]. Their concentration is not influenced by acute hyperglycemia [6], but misleading elevations are possible in hypothyroid patients [8]. Because fructosamines are the product of the spontaneous condensation of glucose with primary amines followed by Amadori rearrangement, their concentrations also depend on protein concentration, turnover and composition [4]. The specific proteins involved are currently not well established, but there is a great deal of evidence that albumin is a major contributor. Studies conducted in dogs identified positive correlations between albumin and fructosamine, but little or no correlation between total protein and fructosamine concentration [4,9]. Because hyperglobulinemia commonly leads to compensatory hypoalbuminemia, fructosamine values are usually low in markedly hyperglobulinemic patients. However, exceptional cases of unknown cause have been reported [9]. Here, markedly elevated fructosamine concentrations in two nondiabetic dogs with a monoclonal IgA-gammopathy are described.
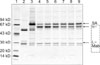
An 8-year-old 21 kg male Pinscher was presented with a 2-week history of vague gastrointestinal signs (inconsistent appetite, flatulence) and lethargy. An extended health check up-profile (IDEXX Vet Med Labor, Germany) revealed unexplainable high fructosamine concentrations as determined based on a colorimetric test conducted using a Roche Hitachi 91 Chemistry Analyzer (Boehringer, Germany; Table 1), despite euglycemia (5.2 mmol/L, reference range 3-5.6 mmol/L) and normal serum thyroxine values (24.5 nmol/L, reference range 19.3-58 nmol/L; measured using a DPC Immulite 1000; Siemens, USA). Severe hyperglobulinemia associated with hypoalbuminemia was also detected (Table 1). The differential diagnosis for hyperglobulinemia in the absence of hyperalbuminemia included polyclonal (chronic inflammation or infection) and monoclonal (lymphoid tumors) gammopathy. The dog was found to be negative for leishmania antibodies (immunofluorescence), ehrlichiosis and anaplasmosis (PCR). No osteolytic lesions or lung metastasis were observed upon radiographic examinations. A bone marrow needle aspiration from the ileum crest was conducted. Due to cluster formation, an exact cell count of the bone marrow aspirate could not be obtained, but plasma cells likely represented > 30% of all nucleated cells. Routine serum electrophoresis on cellulose acetate strips (Interlab Genio Electrophoresis-System, Densitometric Scanning, Elfolab Software; Menarini Diagnostics, Austria) displayed a large peak in the beta/gamma globulin region, comprising about 70% of the overall serum protein (Fig. 1). An additional SDS-PAGE conducted under reducing conditions [5] revealed a monoclonal gammopathy of IgA class (Fig. 2), evidenced by a prominent band at 59 kD (the α- = heavy chain) and a very narrow and distinct light chain of 28 kD. In contrast, light chains of polyclonal immunoglobulins display much larger heterogeneity and thus a much broader band, as seen in the control sample (Lane 3 of Fig. 2) or in IgG preparations (Lane 2 of Fig. 2) [7]. The immunoglobulin class was further confirmed in an immunoblot with an anti-dog IgA antibody (α-chain specific; Bethyl Laboratories, USA). Small amounts of monoclonal antibody could also be detected in the urine. The diagnosis of IgA-multiple myeloma was based on the increased serum concentration of monoclonal immunoglobulins, proteinuria and dominance of plasma cells in the bone marrow. Oral therapy was started with 0.1 mg/kg melphalan hydrochloride (Alkeran; GlaxoSmithKline, Austria) and 0.5 mg/kg prednisolone (Nycomed; Nycomed, Austria) SID. After ten days, the melphalan was reduced to 0.05 mg/kg SID and prednisolone was reduced to 0.5 mg/kg EOD. Upon treatment, the well-being of the dog clearly improved and changes in the albumin and IgA levels were observed in the electrophoretic patterns. Specifically, the IgA concentrations decreased and the albumin band became more prominent (both seen as changes in the thickness and intensity of the respective bands in Fig. 2, Lanes 4-9). The decrease in IgA was associated with a decrease in fructosamine concentration (Table 1, selected days). Fructosamine was positively correlated (Pearson's correlation, 19 measurements) with total protein (r = 0.831, p < 0.001) and globulin (r = 0.888, p < 0.001), but negatively correlated with albumin (r = -0.781, p < 0.001) concentration (Fig. 3). Repeated urine dipstick measurements were negative for glucose, and blood glucose concentrations were within the normal range.
A 14-year-old male Golden Retriever was evaluated because of a three month history of polydipsia, polyuria, lethargy and epistaxis. Except for pale mucous membranes, tachycardia (124 beats per min), a small palpable testical mass and a large palpable spleen (confirmed by ultrasonography: large, dense, inhomogeneous), the clinical examination was unremarkable. Leishmaniasis, anaplasmosis and ehrlichiosis were excluded as described in Case 1. Radiographs of the nose did not show osteolytic lesions or foreign bodies. Platelet counts and blood coagulation times were normal. Notable laboratory findings included non-regenerative anemia (hematocrit 32%, reference range 37-55%), hyperproteinemia, hypoalbuminemia (for proteins see Table 1) and proteinuria (urinary protein/creatinine ratio, 3.95). Despite euglycemia (glucose 4.8 mmol/L) and negative urine glucose dipstick results, the fructosamine levels were high (Table 1). Serum thyroxine was low, but TSH and cholesterol were within the reference range. Multiple myeloma with IgA monoclonal gammopathy was diagnosed based on the results of electrophoresis (similar to Case 1), bone marrow biopsy (bone marrow plasmacytosis, > 30% atypical plasma cells) and fine needle aspiration of the spleen (moderate extramedullary hematopoesis, high number of atypical plasma cells). The IgA concentration was also quantified by an enzyme linked immunosorbent assay (IgA = 1,440 µg/mL, normal range 6.1-973 µg/mL; University of Veterinary Medicine Hannover, Germany) [3]. The testicular mass was confirmed as an interstitial cell tumor. Therapy was started as described above and with the normalisation of protein concentrations, fructosamine returned into the reference range (Table 1).
The high fructosamine concentrations in the dogs observed in this study were unexpected and could not be explained by chronic hyperglycemia or hypothyroidism. Concentrations above 500 µmol/L are not seen in hypothyroid dogs [8] and would indicate poor glycemic control in diabetic dogs. The changes observed in the present study were most likely caused by elevated monoclonal IgA produced by neoplastic plasma cells. In contrast to other studies in dogs, there was a strong negative correlation between albumin and fructosamine concentration in these patients. Despite the increasing albumin concentrations, fructosamines decreased in parallel to clinical and biochemical remission. In human medicine, it is well established that elevated IgA has to be taken into consideration when interpreting fructosamine to avoid misinterpretations. No such effect is seen in patients with elevated polyclonal IgA and monoclonal IgG or IgM myeloma patients [1,2].
The results from the two euglycemic dogs evaluated in this study were parallel to findings in human patients that fructosamine concentrations can be markedly elevated in the presence of a monoclonal IgA-gammopathy. Paraproteinemia should be taken into consideration in dogs with unexplained high fructosamine levels.
Figures and Tables
Fig. 1
Routine serum electrophoresis on a cellulose acetate strip with plastic support of a dog (Case 1) conducted using an automated system.
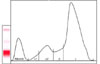
Fig. 2
Serum protein pattern of a dog (Case 1) with monoclonal gammopathy as determined by SDS-PAGE (8 × 6 cm gels with 10-15% polyacrylamide gradient) and detection of the immunoglobulin class. General protein staining (silverstain). Samples from time course (before and during therapy), run under reducing conditions: Lane 1: Molecular weight standard, Lane 2: Dog IgG pure substance, Lane 3: Serum of a healthy dog, Lanes 4-9: Serum samples from time course, at: 23 days before therapy and on the 10th, 24th, 50th, 69th, and 88th day of therapy; samples of this patient were more dilute than the healthy control. SA: serum albumin; MAb: monoclonal antibody (IgA class, consisting of heavy (α-) and light chains (L-)).
References
1. Fujita K, Curtiss LK, Sakurabayashi I, Kameko F, Okumura N, Terasawa F, Tozuka M, Katsuyama T. Identification and properties of glycated monoclonal IgA that affect the fructosamine assay. Clin Chem. 2003. 49:805–808.

2. Fujita K, Kameko F, Kato Y, Fukushima M, Okumura N, Terasawa F, Sugano M, Yamauchi K, Sato H, Kameko M, Sakurabayashi I. Mechanism of IgA-albumin complex formation that affects the fructosamine assay. J Electrophor. 2006. 50:19–23.

3. Griot-Wenk ME, Busato A, Welle M, Racine BP, Weilenmann R, Tschudi P, Tipold A. Total serum IgE and IgA antibody levels in healthy dogs of different breeds and exposed to different environments. Res Vet Sci. 1999. 67:239–243.

4. Kawamoto M, Kaneko JJ, Heusner AA, Feldman EC, Koizumi I. Relation of fructosamine to serum protein, albumin, and glucose concentrations in healthy and diabetic dogs. Am J Vet Res. 1992. 53:851–855.
5. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–685.

6. Marca MC, Loste A, Ramos JJ. Effect of acute hyperglycaemia on the serum fructosamine and blood glycated haemoglobin concentrations in canine samples. Vet Res Commun. 2000. 24:11–16.
7. Miller I, Goldfarb M. Smejkal GB, Lazarev A, editors. Immunoglobulin patterns in health and disease. Separation Methods in Proteomics. 2006. Boca Raton: CRC Taylor & Francis;235–267.

8. Reusch CE, Gerber B, Boretti FS. Serum fructosamine concentrations in dogs with hypothyroidism. Vet Res Commun. 2002. 26:531–536.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download