Abstract
The temperature-sensitive hemagglutinin (Tsh) expressed by strains of avian pathogenic Escherichia (E.) coli (APEC) has both agglutinin and protease activities. Tsh is synthesized as a 140 kDa precursor protein, whose processing results in a 106 kDa passenger domain (Tshs) and a 33 kDa β-domain (Tshβ). In this study, both recombinant Tsh (rTsh) and supernatants from APEC, which contain Tshs (106 kDa), caused proteolysis of chicken tracheal mucin. Both rTsh (140 kDa) and pellets from wild-type APEC, which contain Tshβ (33 kDa), agglutinated chicken erythrocytes. On Western blots, the anti-rTsh antibody recognized the rTsh and 106 kDa proteins in recombinant E. coli BL21/pET 101-Tsh and in the supernatants from APEC grown at either 37℃ or 42℃. Anti-rTsh also recognized a 33 kDa protein in the pellets from APEC13 cultures grown in either Luria-Bertani agar, colonization factor antigen agar, or mucin agar at either 26℃, 37℃, or 42℃, and in the extracts of outer membrane proteins of APEC. The 106 kDa protein was more evident when the bacteria were grown at 37℃ in mucin agar, and it was not detected when the bacteria were grown at 26℃ in any of the culture media used in this study. Chicken anti-Tsh serum inhibited hemagglutinating and mucinolytic activities of strain APEC13 and recombinant E. coli BL21/pET101-Tsh. This work suggests that the mucinolytic activity of Tsh might be important for the colonization of the avian tracheal mucous environment by APEC.
The temperature-sensitive hemagglutinin (Tsh) expressed by avian pathogenic Escherichia (E.) coli (APEC) confers the phenotype mannose-resistant hemagglutination of chicken erythrocytes to bacteria grown at 26℃ on low-osmolarity solid medium [2,12]. The Tsh gene was detected in APEC, but not in E. coli isolated from the faeces of healthy chickens, which suggests that it possibly has a role in the pathogenicity of APEC [4,10]. The deduced sequence of 4.4 kb of Tsh showed homology to the serine-type immunoglobulin A (IgA) proteases of Neisseria gonorrhoeae and Haemophilus influenzae [13]. Since Tsh is secreted in a manner similar to type IgA serine-proteases, it was classified into the subfamily of autotransporter proteins called "serine protease autotransporters of Enterobacteriaceae" (SPATE) [4,7,14].
Maturation of Tsh produces two proteins, a 106 kDa extracellular protein (Tshs) and a 33 kDa outer membrane protein, that corresponding to the β-domain (Tshβ). The 106 kDa protein contains the serine-protease motif, which is also found in secreted IgA proteases, but it does not cleave human IgA or chicken IgA [15], although Tsh cleaves bovine submaxillary gland mucin and coagulation factor V [5]. Tshs adheres to red blood cells, hemoglobin, and the extracellular matrix proteins fibronectin and collagen IV, and it also exerts proteolytic activity against casein [9].
APEC isolated in Brazil often carries the Tsh gene [3] whereas non-pathogenic strains do not carry it, which strengthens the contention that it is an important virulence factor and a possible target for vaccine development. We previously cloned the Tsh gene [14], and in this study we investigated expression and functional characteristics of Tsh from Brazilian isolates of APEC.
APEC 13 strain, serotype O2:H9; APEC 27 strain, serotype O36:H35; and APEC 35, serotype O153:H17 [2], were isolated from colibacillosis lesions in chickens. The E. coli BL 21 Star (DE3) strain (Invitrogen, USA) was transformed with recombinant plasmid pET 101-Tsh, which allowed high-level expression of T7-regulated genes. The strains E. coli BL21 Star (DE3) and E. coli HB101 were used as negative controls.
Bacteria were routinely grown in Luria-Bertani (LB) broth or agar; E. coli BL21 Star (DE3) strain/Tsh was grown in media containing added ampicillin (100 µg/mL). The wild-type strain APEC 13 was grown in agar colonization factor antigen (CFA), and in agar mucin (10 mL 5XM9; 0.5 g porcine gastric mucin, Type III; Sigma, USA; 1 g agar; 50 mL dH2O). Mucin solutions were autoclaved at 121℃ for 15 min prior to use. Samples grown in broth were centrifuged (3,000 × g, 10 min, 4℃), the pellet was frozen at -20℃, and the supernatant was filtered and lyophylized. Samples grown in agar were suspended in 2 mL of 0.85% NaCl, spun (16,460 × g, 40 min, 4℃), the pellet was frozen at -20℃, and the supernatant filtered (0.8 µm and 0.45 µm Millipore filters; Millipore, USA).
The strain E. coli BL 21 Star (DE3)/Tsh was grown in 50 mL of LB broth, at 37℃, and 1 mM of IPTG (Sigma Chemical, USA) was added when the culture reached an OD600 equal to 0.6, followed by an additional 4 h of incubation. The protein was purified from pellets of BL21/Tsh with a ProBond Protein Purification System according the manufacturer's protocol (Invitrogen, USA).
The purified recombinant Tsh (rTsh) protein (100 µg) was resolved by SDS-PAGE (8% gels), and the 140 kDa band was removed and intramuscularly inoculated together with Freund's coadjuvant into Hy-line chickens. Four days after the second boost, the chickens were bled, and the serum was separated, inactivated, and adsorbed into the E. coli BL 21 Star (DE3) strain. Control serum was obtained from non-immunised Hy-line chickens.
Cell lysates and purified protein were suspended in electrophoresis sample buffer (0.025 M Tris-HCl, 2% SDS, 15% glycerol, 2.5% 2-mercaptoethanol, pH 6.8), boiled for 5 min, and electrophoresed on SDS 5~12% polyacrylamide gels (SDS-PAGE). Gels were either stained with Comassie blue or set up for Western blotting. Proteins were transferred onto nitrocellulose membranes (Amershan Pharmacia Biotech, UK) and the membranes were blocked in blocking buffer (PBS with 0.1% Tween 20 and 5% nonfat dry milk) for 1 h at room temperature with agitation. The membranes were washed in PBS-T (PBS with 0.1% Tween 20) and incubated for 1 h in a 1:40,000 dilution of anti-Tsh serum. The membranes were then washed in PBS-T and Tsh protein was detected by an enhanced chemioluminescence Western blotting system (Amersham International, UK). Protein molecular weight markers (BenchMark Pre-Stained Protein Ladder; Invitrogen, USA) were used as standards.
To verify the location of the Tsh protein, APEC 13 strain was grown on CFA broth at 26℃ to induce Tsh protein synthesis. The outer membrane proteins (OMPs) were purified as previously described [6], analysed by SDS-PAGE, and transferred onto nitrocellulose membranes for Western blotting with anti-Tsh serum.
Hemagglutination activity was tested by micro-hemagglutination [12]. Bacteria grown on CFA agar plates at 26℃ for 48 h were harvested and suspended in 0.85% NaCl. When cells were assayed for hemagglutination activity, the suspension of cells was serially diluted in 0.85% NaCl containing 1% methyl-α-D-mannopyranoside (Sigma, USA) to inhibit hemagglutination by type1 pili, and then added to each well of a 96-well round-bottom microplate containing a suspension of fresh chicken erythrocytes. The reactions were incubated for 1 h on ice. Wells containing an even sheet of erythrocytes across the well were considered positive, whereas those containing a small erythrocyte pellet at the bottom of the well were considered negative. To test the presence of inhibitory antibodies in the immune serum, APEC 13, BL21/pET101-Tsh, and BL21 strains were incubated with anti-Tsh serum and control serum for 30 min on ice, and then tested by the micro-hemagglutination assay.
Chicken tracheal mucin was obtained from 10 bled roosters [7]. Their tracheas were removed under aseptic conditions, slit lengthwise, and mucus was collected by gently scraping the surface with a sterile spatula. The mucus was homogenized, filtered, concentrated on Centriprep YM-10 Centrifugal Filter Units (Millipore, USA), and further filtered on 0.45 µm filters (Millipore, USA). Samples of this preparation were plated on LB agar to test for bacterial contamination. Protein content was assayed by Lowry's method [11].
Polyacrylamide gels (8%) co-polymerized with 10 mg (1.25 mg/mL) of mucin were used to assay the mucinase activity of r-Tsh protein and cellular preparations. Porcine gastric mucin (Type III; Sigma-Aldrich, USA), bovine submaxillary mucin (Type I; Sigma Chemical, USA), and chicken tracheal mucin preparation were used as substrates. After electrophoresis, the gels were treated with 1% Triton X-100 for 1 h, rinsed, and incubated for 40 h in mucinase buffer (0.05 M Tris-HCl, pH 8.0; 0.01 M CaCl2; 0.15 M NaCl). The gels were then fixed, and stained by Schiff's periodic acid technique. Inhibition of the mucinolytic activity was tested by incubation of 5 µL anti-Tsh antibody (42 mg/mL) with 10 µL of Tsh (67 µg/mL) for 30 min at room temperature [7].
The mucinolytic activity was also tested in a medium containing 1.5% agarose and 1% of porcine gastric mucin. The preparations were deposited into wells cut in the medium and incubated overnight at 37℃. Zones of clearing were visualised by staining with 0.1% amido-black in 3.5 M acetic acid for 15 min, followed by destaining with 5% acetic acid and 0.5% glycerol for 4 h to overnight.
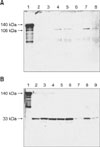
Expression and purification of Tsh was tested in recombinant E. coli BL21/pET 101-Tsh, APEC 13 (O2:H9), APEC 27 (O36:H35), and APEC 35 (O153:H17), in the supernatants and pellets of samples grown in several different media, and at different temperatures. The rTsh purified from resin ProBond was approximately 140 kDa as previously shown [14]. The anti-rTsh antibody recognized the rTsh and a 106 kDa protein (Fig. 1A, Lane 1) on Western blots, but did not recognized a 33 kDa protein (Fig. 1B, Lane 1) in the recombinant E. coli BL21/pET 101-Tsh. Extracts of OMP of APEC 13 also contained the 33 kDa protein that reacted with anti-Tsh antibody.
Supernatants from APEC 13 cultures contained a protein of about 106 kDa when the bacteria were grown at either 37℃ or 42℃ in CFA agar (Fig. 1A, Lanes 4 and 5), although this protein was more evident when the bacteria were grown at 37℃ in mucin agar (Fig 1A, Lane 7). This 106 kDa protein was not detected when the bacteria were grown at 26℃ in any of the culture media used in this study (Fig. 1A, Lanes 2, 3 and 6). A 106 kDa protein was also detected in the supernatants when the bacteria were grown in mucin broth at 37℃ for 48 h. As expected, the 140 kDa protein was not found in the supernatants from APEC 13 cultures.
Pellets from APEC 13 cultures grown in either LB agar, CFA agar, or mucin agar, at either 26℃, 37℃, or 42℃, contained a 33 kDa protein (Fig. 1B, Lanes 2-9). When APEC 13 cultures were grown at 26℃ in LB agar or CFA agar a protein of about 140 kDa was also found (Fig. 1B, Lanes 2 and 4). Pellets from cultures of bacteria grown in liquid medium produced proteins of about the same relative molecular masses as those detected after growth in agar.
Different serotypes of APEC 13 (O2:H9), APEC 27 (O36:H35) and APEC 35 (O153:H17) also produced the 106 kDa protein in the supernatant when grown at 37℃ in mucin-agar, and the 33 kDa protein in the pellet when grown at 26℃ in CFA agar.
Both the recombinant E. coli BL21/pET 101-Tsh expressing Tsh and APEC13 agglutinated chicken erythrocytes at 26℃ that was inhibitable by anti-Tsh antibody, whereas the BL21 strain (Tsh-) was non-agglutinating, indicating that pET101-Tsh contains the structural gene that encodes hemagglutinin Tsh. Supernatants from APEC 13 cultures grown in CFA broth at 37℃, that contain the 106 kDa protein, did not cause hemagglutination. However, OMP extracts from APEC 13 grown at either 26℃ or 37℃, containing the 33 kDa protein, caused hemagglutination which was inhibited by anti-Tsh antibody. The 140 kDa purified rTsh protein caused agglutination of chicken erythrocytes, which was inhibited by anti-Tsh antibody (Table 1).
Concentrated supernatants from APEC 13 cultures were found to have mucinolytic activity, whereas the corresponding pellets did not. A clear halo of protein degradation was observed around of wells with supernatants from APEC 13 grown in agar mucin; this was not observed when the bacteria were grown in either albumin agar or mucin agar supplemented with glucose (Fig. 2). Others strains APEC 27 (O36:H35) and APEC 35 (O153:H17) also showed mucinolytic activity.
Chicken anti-Tsh antibody recognized rTsh protein, but did not recognize any protein from the recipient strain E. coli BL21, showing that it was highly specific for Tsh. This antibody recognized a 106 kDa protein in supernatants from APEC 13, APEC 27, and APEC 35 grown at 37℃, and a 33 kDa in the corresponding pellets, showing that the 140 kDa protein was cleaved into those smaller proteins. This conclusion is due our observations that supernatants from APEC 13 cultures grown at 37℃ do not contain detectable 140 kDa protein, whereas the pellets from cultures grown at 26℃ contain the 140 kDa protein. Thus, these observations suggest that wild-type APEC processes Tsh in a temperature-dependent manner which parallels the original findings in recombinant E. coli K-12 [13,15]. Also, our data are similar to those found by others works that detected the proteins of 33 kDa and 140 kDa in the wild strains grown at 26℃, but the 33-kDa protein only was detect with antibody due small amounts, since it is not expressed at high enough levels [13,15].
Purified rTsh agglutinated chicken erythrocytes; this agglutination was inhibited by anti-Tsh antibody, showing that in aqueous solution the 140 kDa Tsh carries an active agglutinin domain. Since supernatants from APEC cultures, which contain the 106 kDa protein, but do not contain the 140 kDa protein, did not cause hemagglutination. Whereas OMP extracts of bacteria grown at either 26℃ or 37℃, which contain the 33 kDa protein, did cause agglutination, it seems that APEC directed proteolysis of the 140 kDa protein occurred at both growth temperatures. However, an alternative interpretation of these data cannot be excluded. Since APEC 13 cells grown at 26℃ caused hemagglutination and produced the 140 kDa protein whereas their supernatants did not contain detectable amounts of the 106 kDa protein, and given that purified r-Tsh caused agglutination. It is also possible that agglutination caused by cells grown at 26℃ was due to native membrane-bound 140 kDa protein, and that the presence of the 33 kDa protein in their OMP extracts was caused by cleavage during cell disruption and membrane pelleting procedures. Stathopoulos et al. [15] also suggested that a cell-associated form of Tsh is responsible for the hemagglutinin-positive phenotype. This latter alternative implies that APEC directed release of Tshs occurs preferentially at 37℃, and not at 26℃, which could represent an interesting adaptation of releasing proteolytic activity into a medium where it would be useful for bacterial growth.
Both the recombinant E. coli BL21/pET 101-Tsh and the protein r-Tsh cleaved bovine submaxilary mucin, chicken tracheal mucin, and pig gastric mucin. The wild-type strain BL21 did not cleave any of those mucins [8]. In this work, supernatants from APEC 13 cultures grown in agar mucin containing the 106 kDa protein also had proteolytic activity, which was not observed when the bacteria were grown in other agar protein media or in mucin-agar supplemented with glucose. Also, the proteolytic activity is not detected if at this stage the culture is supplemented with additional glucose in Vibrio cholerae [1]. Our data suggests that the mucinolytic activity of Tsh might be important for the colonization of the avian tracheal mucous environment by APEC.
Figures and Tables
Fig. 1
Western blot of fractions from APEC 13 grown in different media and at different temperatures. (A) Supernatant fractions; Lane 1: control, recombinant E. coli BL 21 Star (DE3) strain/Tsh; Lane 2: LB medium, 26℃; Lane 3: CFA, 26℃; Lane 4: CFA, 37℃; Lane 5: CFA, 42℃; Lane 6: mucin, 26℃; Lane 7: mucin, 37℃; Lane 8: mucin, 42℃. (B) Pellet fractions; Lane 1: E. coli BL 21 Star (DE3) strain/Tsh; Lane 2: LB, 26℃; Lane 3: LB, 42℃; Lane 4: CFA, 26℃; Lane 5: CFA, 37℃; Lane 6: CFA, 42℃; Lane 7: mucin, 26℃; Lane 8: mucin, 37℃; Lane 9: mucin, 42℃. The positions of molecular mass markers (kDa) are shown at the left. APEC: avian pathogenic E. coli, LB: Luria-Bertani, CFA: colonization factor antigen.
Acknowledgments
This work was supported by the "Conselho Nacional de Desenvolvimento Científico e Tecnológico" and "Conselho de Aperfeiçoamento de Pessoal de Ensino Superior".
References
1. Benitez JA, Silva AJ, Finkelstein RA. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect Immun. 2001. 69:6549–6553.

2. de Moura AC, Irino K, Vidotto MC. Genetic variability of avian Escherichia coli strains evaluated by enterobacterial repetitive intergenic consensus and repetitive extragenic palindromic polymerase chain reaction. Avian Dis. 2001. 45:173–181.

3. Delicato ER, de Brito BG, Konopatzki AP, Gaziri LC, Vidotto MC. Occurrence of the temperature-sensitive hemagglutinin among avian Escherichia coli. Avian Dis. 2002. 46:713–716.

4. Dozois CM, Dho-Moulin M, Brée A, Fairbrother JM, Desautels C, Curtiss R III. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect Immun. 2000. 68:4145–4154.

5. Dutta PR, Cappello R, Navarro-García F, Nataro JP. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect Immun. 2002. 70:7105–7113.

6. Griffiths E, Stevenson P, Joyce P. Pathogenic Escherichia coli express new outer membrane proteins when growing in vivo. FEMS Microbiol Lett. 1983. 16:95–99.

7. Henderson IR, Navarro-Garcia F, Nataro JP. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998. 6:370–378.

8. Kobayashi RKT, Gaziri LCJ, Venancio EJ, Vidotto MC. Detection of Tsh protein mucinolytic activity by SDS-PAGE. J Microbiol Methods. 2007. 68:654–655.

9. Kostakioti M, Stathopoulos C. Functional analysis of the Tsh autotransporter from an avian pathogenic Escherichia coli strain. Infect Immun. 2004. 72:5548–5554.

10. Maurer JJ, Brown TP, Steffens WL, Thayer SG. The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin Tsh among avian Escherichia coli. Avian Dis. 1998. 42:106–118.

11. Miller GL. Protein determination for large numbers of samples. Analyt Chem. 1959. 31:964.
12. Provence DL, Curtiss R III. Role of crl in avian pathogenic Escherichia coli: a knockout mutation of crl does not affect hemagglutination activity, fibronectin binding, or curli production. Infect Immun. 1992. 60:4460–4467.

13. Provence DL, Curtiss R III. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994. 62:1369–1380.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download