Abstract
The KA1 kainate receptor (KAR) subunit in the substantia gelatinosa (SG) of the trigeminal subnucleus caudalis (Vc) has been implicated in the processing of nociceptive information from the orofacial region. This study compared the expression of the KA1 KAR subunit in the SG of the Vc in juvenile, prepubescent and adult mice. RT-PCR, Western blot and immunohistochemistry analyses were used to examine the expression level in SG area. The expression levels of the KA1 KAR subunit mRNA and protein were higher in juvenile mice than in prepubescent or adult mice. Quantitative data revealed that the KA1 KAR subunit mRNA and protein were expressed at levels approximately two and three times higher, respectively, in juvenile mice than in adult mice. A similar expression pattern of the KA1 KAR subunit was observed in an immunohistochemical study that showed higher expression in the juvenile (59%) than those of adult (35%) mice. These results show that the KA1 KAR subunits are expressed in the SG of the Vc in mice and that the expression level of the KA1 KAR subunit decreases gradually with postnatal development. These findings suggest that age-dependent KA1 KAR subunit expression can be a potential mechanism of age-dependent pain perception.
The substantia gelatinosa (SG) laminar II of the trigeminal subnucleus caudalis (Vc) is a critical site for orofacial nociceptive processing because it receives the synaptic inputs from primary myelinated Aδ and unmyelinated C fibers [38]. The SG neurons function as excitatory and inhibitory interneurons and regulate the output of projection neurons in lamina I and IV, which transmit noxious information to a higher brain center [8,18,23,24,30].
Kainate receptors (KARs) belong to the ionotropic glutamate receptor families, which also include α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-D-aspartate subunits. Native KARs are formed by the heteromeric combination of five subunits, GluR5-7 (GluK1-3) and KA1-2 (GluK4-5). The KARs are expressed in nociceptive pathways including the dorsal root ganglion, spinal cord, thalamus and cortex [45], particularly at the spinal dorsal horn and Vc, which are involved in pain processing [3,7,15]. Among the KAR subunits, GluR5- or GluR6-containing KARs are involved in nociceptive transmission [39,46,47]. Because the KA1 subunit contributes to functional KARs with GluR5/6 subunits, the level of KA1 expression in the SG area can provide key information regarding pain processing. However, there is little information available regarding KA1 KAR subunit expression in the SG area of the Vc in mice. This study examined the KA1 KAR subunit mRNA and protein level in the SG area of the Vc using RT-PCR, Western blotting and immunohistochemistry and compared the expression levels according to the postnatal stage.
All experiments were approved by the Experimental Animal Care and Ethics Committee of Chonbuk National University. The mice (Damul Science, Korea) were housed under 12 h light : 12 h dark cycles (lights on at 07:00 h) with access to food and water ad libitum. Juvenile (postnatal days 7-14), prepubescent (postnatal days 25-35) and adult (postnatal days 42 over) mice were decapitated between 10:00 and 12:00 h, after which their brains were removed rapidly and placed in an ice-cold bicarbonate-buffered artificial cerebrospinal fluid (ACSF) with the following composition (in mM): 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 11 D-glucose, 1.4 NaH2PO4 and 25 NaHCO3 (pH 7.4, bubbled with 95% O2 and 5% CO2). Coronal slices (150-170 µm thickness) containing the rostral part of Vc (1-2 mm from obex) were then cut in ice-cold ACSF using a vibratome (Microm, Germany).
The RT-PCR procedures were conducted as previously described [1]. Briefly, punched samples from the SG of the Vc were harvested from the slice using a specially made 18 gauge needle. The samples were then centrifuged at 12,000 g for 3 min at 4℃ and subsequently homogenized in TRIzol reagent (Invitrogen, USA). Next, 0.2 mL chloroform per 1 mL TRIzol reagent was added and the mixture was shaken for 15 sec and subsequently centrifuged at 12,000 g for 15 min at 4℃. The aqueous phase containing the RNA was then precipitated by mixing with isopropanol (0.5 mL), after which it was centrifuged at 12,000 g for 8 min at 4℃. The RNA pellet was washed with diethylpyrocarbonate (DEPC) water and 75% ethanol (1 mL) and centrifuged at 7,500 g for 5 min at 4℃. The ethanol in the DEPC water was then removed and air-dried for 3 to 5 min. Finally, the pellet was dissolved in DEPC water. The RNA from the sample was quantified using a spectrophotometer (Eppendorf, USA). RNA with the same mass of 1 µg was added to each PCR tube containing the RT pre-mixture (DW 3.6 µL, 3 µg/µL random primers 0.7 µL, 40 U/µL RNaseOUT 0.7 µL (Invitrogen, USA)). The RNA in the RT pre-mixture was mixed gently, heated at 65℃ for 5 min, and then incubated on ice for at least 1 min. The contents of the incubated samples were collected by brief centrifugation, after which 5× first-strand buffer (4 µL), 0.1 M DTT (1 µL), 10 mM dNTP (1 µL), 200 U/µL Superscriptase III (0.5 µL) and nuclease free water (0.5 µL) (Invitrogen, USA) were added. The mixture was then incubated at 25℃ for 5 min and subsequently heated at 50℃ for 60 min. Finally, the reaction was inactivated by heating at 70℃ for 15 min. The RT products were stored at -20℃ in a freezer. The primers of the KA1 subunit (F;5'-ATGCCCCGTGTCTCTGCTCCT-3': R; 5'-TCTGGAGTTGGAACCTGACAAA-3') and GAPDH (F;5'-TTGGCATTGTGGAAGGGCTC-3': R; 5'-TGCTGTTGAAGTCGCAGGAG-3') were designed using the Primer3 program [36]. The PCR reaction of the KA1 subunit and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified using a thermal cycler (MJ Research, USA). cDNA synthesis from the RT sample was amplified in a PCR master mix solution (Promega, USA). Amplification consisted of a denaturation step at 94℃ for 2 min, followed by 30 cycles of 94℃ for 30 sec, 58℃ (KA1) or 62℃ (GAPDH) for 30 sec, and 72℃ for 45 sec, with a final extension step at 72℃ for 10 min. Semi-quantitative RT-PCR analysis of the KA1 subunit expression was estimated after electrophoresis on 2% agarose gels (USB, USA) using ethidium bromide dye. To quantify the expression of the KA1 subunit and GAPDH, the PCR products were analyzed using the density-metric program (Multi Gauge) of a Las-3000 image analyzer (Fuji, Japan) after gel scanning with Gel-Doc 1000 (Bio-Rad, Italy). The expression density of the KA1 subunit PCR products was normalized against GAPDH [9,21,43]. The normalized data of each group (juvenile KA1/GAPDH, prepubescent KA1/GAPDH and adult KA1/GAPDH) were renormalized according to the juvenile density of each group.
Punched samples (n = 4) from the SG of the Vc were lysed in tissue lysis buffer (RIPA buffer; 50 mM Tris-HCl, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 g/mL aprotinin, 1 g/mL leupeptins, 1 g/mL pepstatin A, 100 µg/mL PMSF) on ice for 30 min. For detection of the KA1 subunit, the sample was boiled for 5 min before loading and then separated using 7% SDS-polyacrylamide gels. The sample was then transferred to an immuno-blot PVDF membrane (Bio-Rad, USA). The membranes were blocked for 2 h at RT in 0.05% T-TBS (10 mM Tirs, pH 8.0, 150 mM NaCl, 0.05% Tween 20) containing 5% skim milk (Bio-Rad, USA), after which they were incubated with primary antibodies against the KA1 subunit (1 : 200 in 0.05% T-TBS containing 5% bovine serum albumin, 100~150 kDa; Santa Cruz Biotechnology, USA) and anti-α-Tubulin (1 : 2,000, 50 kDa; Sigma, USA) overnight at 4℃ [27]. After washing three times for 10 min each in 0.05% T-TBS, the membranes were incubated with horseradish peroxidase-conjugated anti-goat antibody (1: 2,000 in 0.05% T-TBS containing 5% bovine serum albumin; Santa Cruz Biotechnology, USA). The membranes were then washed with 0.05% T-TBS and exposed to X-ray film. The protein expression of the KA1 subunit was analyzed using a density-metric Multi Gauge program in the Las-3000 image analyzer (Fuji, Japan) after scanning the X-ray films. The expression density of the KA1 subunit was normalized to anti-α-tubulin. The normalized data from each group (juvenile KA1/α-Tubulin, prepubescent KA1/α-Tubulin and adult KA1/α-Tubulin) was then renormalized according to the juvenile density of each group.
The brains of juvenile and adult groups were removed rapidly (each n = 4), placed in 4% paraformaldehyde and fixed overnight at 4℃. The brains were then dehydrated in 70%, 95% and 100% ethanol two times each for 45 min at RT, after which the samples were cleaned in xylene three times for 30 min at RT. The cleaned brains were then embedded in paraffin. Next, 5 µm tissue sections were mounted on ProbeOn Plus Microscope Slides (Fisher Biotech, USA). Each slide was then deparaffinized in xylene and rehydrated in ethanol, after which it was rinsed in DW and the endogenous peroxide activity was suppressed by incubation in 3% H2O2 for 5 min. The slide was then rinsed in PBS and incubated in blocking normal serum (Vector Laboratories, USA) for 1 h at RT followed by incubation with the primary antibody in blocking normal serum (1 : 50) and anti-KA1 overnight at 4℃. After washing with PBS, the slides were reacted in biotinylated pan-specific antibody anti-mouse/rabbit/goat IgG (H + L) (Vector Laboratories, USA) for 30 min at RT. Subsequently, the slide was rinsed in PBS and reacted in the strepavidin-peroxidase complex (Vector Laboratories, USA) for 30 min. The slides were rinsed again in PBS, stained using an AEC peroxidase substrate kit (Vector Laboratories, USA) and counterstained with hematoxylin. The slide was then evaluated based on random areas under a microscope. The negative control was examined using an identical method, but without the primary antibody. The KA1 subunit detected in the SG was examined at three relative expression levels (0%, 50%, 100%) by a blind test and samples with over 50% were used for analysis.
Fig. 1A provides an example of the expression of KA1 subunit mRNA in the SG of the Vc in juvenile, prepubescent and adult mice. The housekeeping gene, GAPDH, was used to confirm identical loading on the agarose gel. Fig. 1B shows the relative KA1 mRNA expression levels. The relative mRNA expression levels of the KA1 subunit in the prepubescent (0.68 ± 0.086, n = 5) and adult (0.54 ± 0.061, n = 8) mice were significantly lower than that of the juvenile mice (n = 8). However, there was no significant difference in the expression levels of prepubescent and adult mice.
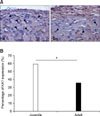
Western blot of a punched sample (n = 4) from the SG of the Vc was conducted to determine if the KA1 protein level of the KAR subunit also showed a similar pattern to that of mRNA expression. Fig. 2 provides an example of the protein expression of the KA1 subunit. The KA1 subunit protein level decreased with increasing postnatal development stage. The housekeeping gene encoding α-Tubulin was used as a reference to normalize the expression level of each sample. As shown in Fig. 2, the protein levels in the prepubescent (0.33 ± 0.19) and adult mice (0.34 ± 0.14) were lower than that of juvenile mice showing a similar pattern of mRNA expression. Immunohistochemistry was conducted to confirm the postnatal change in KA1 containing KAR expression. As shown in Figs. 1 and 2, KA1 mRNA and protein expression levels were similar in the prepubescent and adults. Therefore, this study compared the KA1 receptor immunoreactivity between juvenile and adult mice. Figs. 3Aa and 3Ab show the immunoreactivity of the KA1 subunit in a coronal slice including the SG neurons of the Vc in juvenile and adult mice, respectively. The KA1 subunits were more highly expressed in the juvenile laminar II containing SG neurons (85/143, 59%) of the Vc than in adults (35/99, 35%). The KA1 subunits were stained a reddish color. Analysis of the counted SG neurons using the SPSS program (SPSS, USA) showed a significant difference in the expression level of the KA1 subunits between the juvenile and adult mice (Fig. 3B). A significant difference was also observed between juvenile and adult mice. However, there was no significant difference in the expression of the KA1 subunit between males and females.
KARs are expressed in the spinal dorsal horn [11,14,16,31,32,37] and play an important role in sensory transmission. For example, KA can release GABA/glycine and glutamate [17] at presyanptic terminals in the spinal dorsal horn. In addition, the KARs are located on the postsynaptic membrane of the spinal dorsal horn neurons [22] and in identified glial cells in a rat spinal cord slice [48]. Functional KARs are expressed as tetrameric combinations of subunits, i.e. dimers of dimers. The subtypes GluR5-7 form homomeric or heteromeric combinations [5,40], whereas the KA1 and KA2 subtypes form exclusively heteromeric functional KA receptors by partnering-up with GluR5 to 7, but not with the opposite KA1/2 [20]. Although many studies have reported the expression of KARs in the spinal dorsal horn, which is involved in pain processing, few investigations of the trigeminal subnucleus caudalis, which is involved in orofacial pain processing, have been conducted.
The results of this study indicated that the expression level of the KA1 KAR subunit mRNA in the SG of the Vc was lower in adult mice than juvenile mice. A similar pattern of postnatal dependent KA1 KAR subunit mRNA expression was reported by Stegenga and Kalb [41] in the rat spinal cord using an in situ hybridization study. They found moderate expression of the KA1 subunit mRNA at postnatal day (PND) 2, but no mRNA for the KA1 KAR subunit transcript was detected in the PND22 spinal cord. Moreover, in the rat hippocampus, including the CA2, CA3 and CA4 regions, KA1 subunit mRNA expression increased transiently over the birth levels, after which it is decreased gradually to PND35 [35].
Based on the principle of central dogma, mRNA is translated to proteins. In the Western blot study, the KA1 subunit was expressed in the juvenile SG of the Vc. However, the expression level was quite low in prepubescent and adult mice. A similar pattern was also observed in the immunohistochemical study. Although there was an age-dependent decrease in KA1 subunit expression, no difference was observed in the expression of the KA1 subunit between males and females. These results are consistent with the expression level of the KA1 KAR subunit mRNA. Since co-expression of the neuronal marker with the KA1 subunit on SG was not examined, it is not clear if all the immunoreactive cells are neurons. However, Hantman et al. [10] reported that NeuN (a neuronal marker)-immunoreactive cells are expressed extensively in the boundary of laminar IIo and laminar IIi.
The neurons expressing high-affinity KAR subunits in the superficial laminae of the dorsal horn of the spinal cord were demonstrated by in situ hybridization [42]. Lu et al. [25] reported the expression of the KA1 KAR subunit at the postsynaptic and presynaptic sites in the spinal cord. Presynaptic immunoreactivity for KA1 was also observed in the terminals of the primary afferent fibers to the superficial laminae of the dorsal horn [26]. However, it is unclear if the postnatal decrease in KA1 subunit expression occurred on the presynaptic terminals, postsynaptic soma/dendrites or glial cells because RT-PCR and Western blotting were conducted in punched samples from the SG area. However, the immunohistochemical data showed that a postnatal decrease in KA1 subunit expression may occur on the postsynaptic membrane, at least in part. Patch clamp studies are powerful tools for identifying postnatal changes in functional KA1 containing KAR expression on pre- or post-synaptic neurons. However, to date, the physiological roles of native KA1 containing KARs have been difficult to study due to the lack of selective agonists or antagonists for the KA1 subunit. Future studies should examine whether age-dependent functional changes in the KA1 subunit also occur when specific KA1 agonists become available.
Expression of the KA1 subunit can be altered by various stimuli. Chen et al. [2] reported that KA1 subunit expression was upregulated dramatically by KA injection into the CA1 region of the hippocampus in adult mice. KA1 expression can also be changed by stress-related hormone levels in the CA3 and dentate gyrus regions of the dorsal hippocampus [13]. Although studies using gene knock out techniques have shed light on the functions of the KA2, GluR5, GluR6 and GluR7 subunits [4,28,29,33], the precise physiological and pathological functions of the KA1 subunit are not clear [12,19,20].
It has been reported that the degree of pain perception can be changed by the postnatal stages. For example, the withdrawal threshold to mechanical stimulation was lowest in 2-week old rats, intermediate in 4-week old rats and highest in 16 week-old animals [34]. Similarly, Walker et al. [44] reported that the baseline mechanical withdrawal threshold increased with increasing postnatal stage. These results suggest an age dependent change in pain processing. Recently, Fernandes et al. [6] generated KA1 subunit knockout mice and reported that KARs do not function efficiently as ligand-gated ion channels without high affinity subunits (KA1 and KA2), suggesting that high-affinity KAR subunits are essential for ionotropic glutamate receptor signaling.
This study showed a difference in the expression of the KA1 subunit on the SG neurons of the Vc in juvenile and prepubescent/adult mice through RT-PCR, Western blot and immunohistochemistry. Since the KA1 subunit contains a high affinity agonist-binding site, changes in expression may affect the binding of the receptors to KA. Overall, the different level of KA1 KARs expression according to postnatal stages suggests that age-dependent KA1 KAR subunit expression can be a potential mechanism of age-dependent pain perception.
Figures and Tables
Fig. 1
(A) Expression of the KA1 subunit mRNA in the substantia gelatinosa (SG) of the trigeminal subnucleus caudalis (Vc). The KA1 subunit is more strongly expressed in juvenile than prepubescent or adult mice. (B) The relative expression of normalized KA1 mRNA in juvenile, prepubescent and adult mice. *p<0.05. NS: not significant, GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
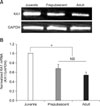
Fig. 2
KA1 subunit protein expressed in the punched lamina II containing SG neurons. Expression of the KA1 subunit decreased gradually with postnatal development.
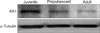
Fig. 3
The KA1 subunit was expressed differently in juvenile and adult mice. (A) Immunoreactivities for the KA1 subunit containing kainate receptors were observed in the SG of the Vc in juvenile (Aa) and adult (Ab) mice. The closed and open arrowheads indicate intense and weak expression of KA1 subunits, respectively. (B) The percentage of neurons expressing the KA1 subunit in the juveniles and adults. *p<0.05. Immunohistochemical staining and couterstain with hematoxylin, ×400.
Acknowledgments
This study was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-E00224).
References
1. Bureau I, Dieudonne S, Coussen F, Mulle C. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci USA. 2000. 97:6838–6843.

2. Chen ZL, Yu H, Yu WM, Pawlak R, Strickland S. Proteolytic fragments of laminin promote excitotoxic neurodegeneration by up-regulation of the KA1 subunit of the kainate receptor. J Cell Biol. 2008. 183:1299–1313.

3. Chiang CY, Hu JW, Sessle BJ. NMDA receptor involvement in neuroplastic changes induced by neonatal capsaicin treatment in trigeminal nociceptive neurons. J Neurophysiol. 1997. 78:2799–2803.

4. Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2-/- mice. J Neurosci. 2003. 23:422–429.

5. Cui C, Mayer ML. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J Neurosci. 1999. 19:8281–8291.

6. Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009. 63:818–829.

7. Furuyama T, Kiyama H, Sato K, Park HT, Maeno H, Takagi H, Tohyama M. Region-specific expression of subunits of ionotropic glutamate receptors (AMPA-type, KA-type and NMDA receptors) in the rat spinal cord with special reference to nociception. Brain Res Mol Brain Res. 1993. 18:141–151.

8. Gobel S, Falls WM, Bennett GJ, Abdelmoumene M, Hayashi H, Humphrey E. An EM analysis of the synaptic connections of horseradish peroxidase-filled stalked cells and islet cells in the substantia gelatinosa of adult cat spinal cord. J Comp Neurol. 1980. 194:781–807.

9. Guo W, Zou S, Tal M, Ren K. Activation of spinal kainate receptors after inflammation: behavioral hyperalgesia and subunit gene expression. Eur J Pharmacol. 2002. 452:309–318.

10. Hantman AW, van den Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. J Neurosci. 2004. 24:836–842.

11. Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990. 5:255–266.

13. Hunter RG, Bellani R, Bloss E, Costa A, McCarthy K, McEwen BS. Regulation of kainate receptor subunit mRNA by stress and corticosteroids in the rat hippocampus. PLoS One. 2009. 4:e4328.

14. Hwang SJ, Pagliardini S, Rustioni A, Valtschanoff JG. Presynaptic kainate receptors in primary afferents to the superficial laminae of the rat spinal cord. J Comp Neurol. 2001. 436:275–289.

15. Kalb RG, Fox AJ. Synchronized overproduction of AMPA, kainate, and NMDA glutamate receptors during human spinal cord development. J Comp Neurol. 1997. 384:200–210.

16. Kerchner GA, Wang GD, Qiu CS, Huettner JE, Zhuo M. Direct presynaptic regulation of GABA/glycine release by kainate receptors in the dorsal horn: an ionotropic mechanism. Neuron. 2001. 32:477–488.

17. Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. Presynaptic kainate receptors regulate spinal sensory transmission. J Neurosci. 2001. 21:59–66.

18. Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978. 177:417–434.

19. Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003. 4:481–495.

21. Leszek P, Korewicki J, Klisiewicz A, Janas J, Biederman A, Browarek A, Charlemagne D, Trouvé P. Reduced myocardial expression of calcium handling protein in patients with severe chronic mitral regurgitation. Eur J Cardiothorac Surg. 2006. 30:737–743.

22. Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999. 397:161–164.

23. Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III-V). J Comp Neurol. 1988. 267:172–189.

24. Light AR, Trevino DL, Perl ER. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979. 186:151–171.

25. Lu CR, Willcockson HH, Phend KD, Lucifora S, Darstein M, Valtschanoff JG, Rustioni A. Ionotropic glutamate receptors are expressed in GABAergic terminals in the rat superficial dorsal horn. J Comp Neurol. 2005. 486:169–178.

26. Lucifora S, Willcockson HH, Lu CR, Darstein M, Phend KD, Valtschanoff JG, Rustioni A. Presynaptic low- and high-affinity kainate receptors in nociceptive spinal afferents. Pain. 2006. 120:97–105.

27. Monnerie H, Le Roux PD. Glutamate receptor agonist kainate enhances primary dendrite number and length from immature mouse cortical neurons in vitro. J Neurosci Res. 2006. 83:944–956.

28. Mulle C, Sailer A, Pérez-Otaño I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998. 392:601–605.

29. Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron. 2000. 28:475–484.

30. Pan YZ, Pan HL. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol. 2004. 91:2413–2421.

31. Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993. 11:1069–1082.

32. Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994. 349:85–110.

33. Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2007. 104:12181–12186.

34. Ririe DG, Eisenach JC. Age-dependent responses to nerve injury-induced mechanical allodynia. Anesthesiology. 2006. 104:344–350.
35. Ritter LM, Vazquez DM, Meador-Woodruff JH. Ontogeny of ionotropic glutamate receptor subunit expression in the rat hippocampus. Brain Res Dev Brain Res. 2002. 139:227–236.

36. Rozen S, Skaletsky H. Misener S, Krawetz S, editors. Primer3 on the WWW for general users and for biologist programmers. Bioinformatics Methods and Protocols: Methods in Molecular Biology. 2000. Totowa: Humana Press;365–386.

37. Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993. 4:1263–1265.

38. Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000. 11:57–91.

39. Simmons RM, Li DL, Hoo KH, Deverill M, Ornstein PL, Iyengar S. Kainate GluR5 receptor subtype mediates the nociceptive response to formalin in the rat. Neuropharmacology. 1998. 37:25–36.

40. Sommer B, Burnashev N, Verdoorn TA, Keinänen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992. 11:1651–1656.

41. Stegenga SL, Kalb RG. Developmental regulation of N-methyl-D-aspartate- and kainate-type glutamate receptor expression in the rat spinal cord. Neuroscience. 2001. 105:499–507.

42. Tölle TR, Berthele A, Zieglgänsberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci. 1993. 13:5009–5028.

43. Ullal G, Fahnestock M, Racine R. Time-dependent effect of kainate-induced seizures on glutamate receptor GluR5, GluR6, and GluR7 mRNA and protein expression in rat hippocampus. Epilepsia. 2005. 46:616–623.

44. Walker SM, Howard RF, Keay KA, Fitzgerald M. Developmental age influences the effect of epidural dexmedetomidine on inflammatory hyperalgesia in rat pups. Anesthesiology. 2005. 102:1226–1234.

45. Wu LJ, Ko SW, Zhuo M. Kainate receptors and pain: from dorsal root ganglion to the anterior cingulate cortex. Curr Pharm Des. 2007. 13:1597–1605.

46. Xu H, Wu LJ, Zhao MG, Toyoda H, Vadakkan KI, Jia Y, Pinaud R, Zhuo M. Presynaptic regulation of the inhibitory transmission by GluR5-containing kainate receptors in spinal substantia gelatinosa. Mol Pain. 2006. 2:29.

47. Youn DH, Randic M. Modulation of excitatory synaptic transmission in the spinal substantia gelatinosa of mice deficient in the kainate receptor GluR5 and/or GluR6 subunit. J Physiol. 2004. 555:683–698.

48. Ziak D, Chvátal A, Syková E. Glutamate-, kainate- and NMDA-evoked membrane currents in identified glial cells in rat spinal cord slice. Physiol Res. 1998. 47:365–375.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download