Abstract
This study was carried out to investigate fifteen cases of acute lethal infection of calves (≤ 4 months of age) by the protozoan parasite Theileria (T.) annulata in the south of Portugal. Calves developed multifocal to coalescent nodular skin lesions, similar to multicentric malignant lymphoma. Infestation with ticks (genus Hyalomma) was intense. Theileria was seen in blood and lymph node smears, and T. annulata infection was confirmed by isolation of schizont-transformed cells and sequencing of hypervariable region 4 of the 18S rRNA gene. At necropsy, hemorrhagic nodules or nodules with a hemorrhagic halo were seen, particularly in the skin, subcutaneous tissue, skeletal and cardiac muscles, pharynx, trachea and intestinal serosa. Histologically, nodules were formed by large, round, lymphoblastoid neoplastic-like cells. Immunohistochemistry (IHC) identified these cells as mostly CD3 positive T lymphocytes and MAC387 positive macrophages. A marker for B lymphocytes (CD79αcy) labeled very few cells. T. annulata infected cells in these nodules were also identified by IHC through the use of two monoclonal antibodies (1C7 and 1C12) which are diagnostic for the parasite. It was concluded that the pathological changes observed in the different organs and tissues were caused by proliferation of schizont-infected macrophages, which subsequently stimulate a severe uncontrolled proliferation of uninfected T lymphocytes.
Various cases of multifocal to coalescent nodular skin lesions and lymph node enlargement, similar to multicentric malignant lymphoma, appeared in very young calves in the south of Portugal. All cases were identified within a short time period, starting from the end of 1999 and progressing through to November 2007. Veterinarians working in the area commented that diseased calves displaying similar pathology had been observed in the past, although less frequently, and that no diagnosis had ever been achieved.
As outlined below, the results of our investigation strongly support the conclusion that death of the affected calves was the result of acute tropical theileriosis, a disease caused by Theileria (T.) annulata, a protozoan parasite that has a biphasic life cycle. The first phase of the life cycle occurs in the arthropod vector, ticks of the genus Hyalomma, while the second phase occurs in the definitive mammalian host, domesticated and wild ruminants [23]. Tropical theileriosis is highly prevalent in Africa, southern Europe, the Near East and Far East and Central Asia, and has had considerable economic impact on livestock production due to the high morbidity and mortality of the disease [5,6].
Infection of cattle occurs through inoculation of sporozoites during feeding by the infected tick. Sporozoites mostly infect macrophages [8,9,15,24,32,39], but can also infect B lymphocytes [14,24,32,39]. The parasite continues its development in the cytoplasm of host cells, differentiating via the trophozoite into the multinucleated macroschizont (schizogony), which has the ability to stimulate cell division of the infected leukocyte. The parasite divides in synchrony with the daughter leukocytes resulting in a clonal expansion of cells. The initial phase of expansion occurs in the lymph node draining the site of tick attachment, but as the disease progresses, infected leukocytes spread to a range of different organs and tissues, where they stimulate the division of uninfected cells while continuing to multiply as parasite infected cells [15,16,24,28,33].
Following expansion of infected leukocytes, host cell division slows down and merozoites are formed. Merozoites are then released as the leukocyte is destroyed, infect the red blood cells and transform into piroplasms (gametocytes), which are the infective form for the tick. The sexual phase of the life cycle of the T. annulata proceeds in the digestive system of the infected tick and gives rise to the production of sporozoites in the salivary glands of the tick [23].
Theileria spp. infection can cause acute, subacute or chronic disease pathology [16]. In endemic areas, affected indigenous breeds present mainly a subacute condition and are resistant to re-infection upon recovery. Imported breeds or crossbred (imported/native) animals are more susceptible and can suffer high mortality rates [5,27]. Infection by Theileria spp. in Portugal has not been subject to recent study; the only reference published on T. annulata in Portugal dates to 1945 [21]. Tropical theileriosis is generally considered by Portuguese parasitologists to be an endemic tick borne infection of cattle with mild/moderate pathology, primarily due to regular identification of the parasite in blood smears collected from asymptomatic animals. In contrast, we provide conclusive evidence that T. annulata was the causative agent of cases of a fatal disease recently identified in Portugal, and discuss how parasite infections could generate the pathological changes that characterised these cases.
From December 1999 to November 2007, 15 calves (numbered 1 to 15) younger than four months old, showing signs of an acute disease syndrome leading to death or euthanasia, were identified from the southern region of Portugal (Table 1). All animals were born on the farms where they lived and all of these farms were registered as free of bovine leukosis virus. Distance between affected farms ranged from 20 to 100 km. Some cases came to the authors' knowledge only after death or euthanasia, which limited sampling for further analysis.
Peripheral blood smears were obtained in seven cases (animals 4, 7, 8, 9, 10, 12 and 14), and lymph node fine needle aspirates were obtained in six cases (animals 7, 8, 9, 10, 12 and 15). All smears were stained using Giemsa and assessed for parasitaemia by examining 10 random fields (×1,000). Blood samples for hemograms were collected from animals 4 and 12. The hemogram of calf 4 was performed at the Veterinary Laboratory of Clinical Analysis LABVET (Portugal), and blood analysis of animal 12 was performed at the Laboratory of Clinical Analysis of the Veterinary Hospital of the University of Évora (Portugal).
All calves were necropsied and samples of organs and tissues collected for histopathology. The samples were fixed in 10% neutral-buffered formalin and processed for examination by standard light microscopy techniques. Paraffin sections were cut at 3 µm and stained with hematoxylin - eosin (H&E) and Giemsa. During necropsy of the euthanized animal 4, a bone marrow aspiration was performed to provide information on hematopoietic cells. Giemsa stained smears were examined at the Laboratory of Clinical Analysis of the Faculty of Veterinary Medicine of the Technical University of Lisbon (Portugal).
The indirect immunoperoxidase method (streptavidin-biotin-peroxidase) was used for immunolabeling sections of skin and skeletal muscle nodules and lymph nodes using two monoclonal primary antibodies, anti-CD79αcy (clone HM57; Dako, USA) and anti-human macrophage (clone MAC387; AbD Serotec, UK), and one polyclonal primary antibody anti-CD3-(Dako, USA) for the detection of B lymphocytes, macrophages and T lymphocytes, respectively. For immunolabeling T. annulata infected cells two monoclonal antibodies (mAbs), 1C7 and 1C12, previously shown to be specific for the macroschizont stage of the parasite were used [17,36,37]. Tissue sections were rehydrated and treated with 3% hydrogen peroxide in water for 20 min to eliminate endogenous peroxidase activity. Antigen retrieval was performed by microwave irradiation (Samsung TDS; Samsung, Korea) in 10 mM citrate buffer at pH 6.0 (MAC387, 1C7 and 1C12) or in 1 mM EDTA at pH 9.0 (anti-CD79αcy and anti-CD3). The slides were irradiated at maximum power (800 W) until the solution was boiling, then the power was lowered to 80% of the maximum for an additional 10 min followed by rinsing with TBS. Slides were incubated with primary antibodies anti-CD79αcy, anti-CD3, 1C7 and 1C12 for 1 h at room temperature or overnight at room temperature for MAC387. Dilutions were 1 : 100 for MAC387 and anti-CD3, 1 : 50 for anti-CD79αcy and neat culture supernatant for mAbs 1C7 and 1C12.
The SuperPicture Polymer (Zymed Laboratories, USA) detection system was used according to the manufacturer's instructions and the labeling was visualized by staining with 3.3'-diaminobenzidine (SuperPicture Polymer; Zymed Laboratories, USA) for 8-10 min. Finally, the slides were counter-stained with Mayer's haematoxylin and mounted according to standard techniques. Negative controls were performed by replacing the primary antibody with TBS. Positive controls consisted of bovine tissues known to be reactive for each marker, using the lymph node for B and T cells and the spleen for macrophages. The level of cellular staining was evaluated in a semiquantitative manner [negative (-); <10% (+); 10-25% (++); 25-50% (+++); >50% (++++)] by examination of the entire histological sections.
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized (20 IU/mL) blood (animal No. 8 and 9) by centrifugation over Ficoll-Paque PLUS (Amersham Biosciences). The PBMC were then washed three times with Hanks' balanced saline solution and the final pellet resuspended in culture medium (RPMI 1640 supplemented with 20% foetal bovine serum, 2 mM L-glutamine, 100 UI/mL penicillin, 100 µg/mL streptomycin). PBMC suspensions (5 × 106 cells/mL) were seeded in plastic tissue culture flasks and incubated at 37℃, in 5% CO2 and >80% humidity. After 48 h, non-adherent cells were gently removed and fresh culture medium added. This procedure was repeated until the loosely adherent cells nearly covered the flask. Subsequently, cell cultures were passaged two to three times a week as described [34]. Total DNA extracts from schizont-infected cells were prepared using a commercial kit (High Pure Viral Nucleic Acid Kit; Roche, Switzerland), following the manufacturer's instructions. For PCR amplification of the hypervariable region 4 of the small subunit 18S ribosomal RNA gene, a pair of primers complementary to sequences flanking this region and are conserved across Babesia and Theileria species were designed using the software programs primer3 [31] and BLAST [41]. PCR reactions were performed in a 50 µL mixture containing ×10 PCR buffer and 2.5 units Taq Platinum DNA polymerase (Invitrogen, USA), 1 mM MgCl2, 0.2 mM each dNTP, 0.5 µM of each forward (5' CAATCCTGACACAGGGAGGT 3') and reverse (5' TGATCGTCTTCGATCCCCTA 3') primers. The reaction mixture was incubated in a thermal cycler at 94℃ for 3 min and cycled: 30 times 94℃, 30 sec; 60℃, 30 sec; 72℃, 1 min, with a final 7 min extension at 72℃. The product was detected by agarose gel electrophoresis using ethidium bromide, and the nucleotide sequence of the amplified fragment determined (STAB Vida, Portugal). The BLAST program (NIH, USA) was used to analyze sequence identity [41].
All animals suffering from disease as reported in this study died or were euthanized one week to fifteen days after the onset of clinical symptoms because there was no possibility of recovery. Affected animals displayed emaciation, anaemia, unilateral or bilateral exophthalmia, petechiae in conjunctiva, oral and nasal mucosa, and occasionally in the pinnae. Widespread subcutaneous nodules with a 0.5 to 3.0 cm diameter were also detected, as well as enlarged superficial lymph nodes, particularly the submandibular, the retropharyngeal, and sometimes the prescapular. All animals had a high level of infestation by ticks of the genus Hyalomma.
Results of the hemograms of animals 4 and 12 revealed anaemia (red blood cells 2.52 × 106/µL and 2.75 × 106/µL; hemoglobin 3.6 g/dL and 3.3 g/dL, respectively). Bone marrow analysis of animal 4 showed marked erythroid hypoplasia (rubriblast and prorubricytes 0% and rubricytes and metarubricytes 0.7%) and the presence of a high number of lymphoid-like cells together with several criteria indicating malignancy: anisocytosis, anisokaryosis and multiple nucleoli. Some of these cells presented Theileria-like organisms within their cytoplasm.
Schizont-infected lymphoblastoid cell lines were successfully initiated from cases 8 and 9, registered in farms 20 km apart with an interval of 14 months (Table 1), and were used to identify the parasite species responsible for transformation of the bovine cells. PCR amplification of the hypervariable region 4 of the small subunit 18S ribosomal RNA gene resulted in a single band of the predicted size, 500 bp, as observed by agarose gel electrophoresis. Amplified fragments generated from three independent PCR reactions, one from culture 8 and two from culture 9, were directly sequenced in both directions and the sequence of the central 400 bp region, read in both directions for all 3 amplicons, was deposited in GenBank under the accession number GQ465761. Searching for homology in nucleotide databases generated 100% identity with the corresponding 18s rRNA sequence of T. annulata, Ankara strain (accession NW_001091929) and 99-100% identity with sequences representing other T. annulata isolates deposited in GenBank. Comparison with sequences derived from other species of Theileira that infect bovines, gave the highest level of identity with T. parva (97%) followed T. taurotragi (96%).
Upon necropsy, external examination identified similar changes to those described for live diseased animals in the field. Calves 9 and 11 had jaundice. Many white nodules (sometimes haemorrhagic or with a haemorrhagic halo) 0.2-3 cm in diameter with a compact and uniform cut surface were identified in the skin and subcutaneous tissue of all carcasses (Fig. 2A). Similar nodules were observed in skeletal muscles (particularly in the tongue, intermandibular muscles and cervical muscles), abdominal fat, omentum and intestinal serosa. Some calves exhibited these nodules in the pharynx (Fig. 2B), trachea, myocardium, pituitary gland, thymus and retrobulbar tissues. Widespread enlarged lymph nodes were identified in all cases. These presented an oedematous, haemorrhagic or alternatively a homogeneous, firm and pale yellow cut surface. Other macroscopic changes included hydrohemothorax, congestion and oedema of the lungs, a friable spleen and an enlarged liver.
The nodules described in the various tissues were found to be formed by round cells, similar to lymphoid neoplastic cells with moderate pleomorphism (Fig. 3A). In the lymph nodes and spleen, moderate infiltration by these cells was accompanied by hypoplasia of the lymphoid tissue. Some lymph nodes also showed heavy diffuse lymphocytic infiltration with no clear cortico-medullary demarcation. In the skin, the infiltration rarely reached the superficial dermis and did not infiltrate the epidermis, being mostly limited to the middle and deep dermis.
The neoplastic-like cells had a cloudy cell membrane, a scarce and slightly basophilic cytoplasm, a high nucleus/cytoplasm ratio, occasionally indented nuclei, chromatin forming granules near the nuclear membrane, and signs of cariorrexis (Fig. 3B). The cells also displayed moderate anisocaryosis and had a high mitotic index, with 2 to 3 mitoses per field (×400). Occasionally, macroschizonts of T. annulata detected by Giemsa stain were identified in the cytoplasm of these cells.
In almost all the nodules described above, the cell population was formed by CD3 positive T lymphocytes and macrophages labelled by MAC387 (Table 3; Figs. 3C and D). Cells labeled by these antibodies displayed strong cytoplasmic staining. Some CD3 positive cells also presented cell membrane staining. Few B lymphocytes positive for CD79αcy were observed, and they were interspersed amongst T lymphocytes and macrophages. Most lymphocytes identified in lymph nodes were CD3 positive T cells (Table 3). The expected labeling profile was obtained in all positive controls and specificity was confirmed by negative staining when the antibodies were replaced by TBS.
When using mAbs 1C7 and 1C12, T. annulata macroschizonts were detected in 25-50% of the neoplastic-like cells identified in histological lesions and occasionally in more than 50% of these cells (Table 3; Figs. 4A and B).
Infection with bovine leukosis virus (BLV) was disregarded from the beginning because the farms were and remain officially free of the virus. Furthermore, the age of the animals and the clinical presentation were not compatible with BLV [40]. Based on this conclusion we postulated that the animals could have suffered from bovine sporadic leukosis (BSL), a disease of unknown aetiology. However, the frequency of the first cases identified in a limited geographic area, together with the clinical and pathological observations did not completely match a diagnosis of BSL [40]. BSL affects animals under six months old, and is characterized by the presence of white nodules with different sizes, located mainly in the kidney, thymus, liver and spleen, and by exuberant hypertrophy of most of the internal and surface lymph nodes [40]. In addition, lesions in the animals examined in this study were different from those reported in cases of BSL, as the kidneys, liver or spleen, were rarely affected and the nodular lesions were mainly located in other organs. These findings led us to consider other possible causes, of which infection by Theileria parasites appeared the most probably, since all the lesions identified were similar to those described by other authors for Tropical theileriosis and East Coast fever [12-16,25]. Furthermore, all the diseased animals were heavily infested with Hyalomma ticks, the known vector of T. annulata [23]. Further work was carried out to confirm whether these animals were infected with T. annulata and to verify the relationship between infection by this protozoan and the lesions characterised in the diseased animals.
In addition to signs similar to malignant lymphoma, tropical theileriosis is known to cause significant anemia [16]. The results of the hemograms and assessment of parasitaemia confirmed the presence of anemia observed in the field and are in agreement with reports on tropical theileriosis by other authors [14,15]. Moreover, the bone marrow test of animal 4 confirmed the presence of lymphoid cells with similar characteristics to those described for the different nodular lesions, and also indicated the presence of intracellular Theileria-like organisms. The latter was confirmed by using two monoclonal antibodies diagnostic for the macroschizont of T. annulata [17,31,36,37] that clearly detected the parasite in the lesions of the skin, skeletal muscle and lymph nodes. Nodular lesions, as identified by immunohistochemistry, were mainly formed by T lymphocytes and macrophages, 25 to 50% of which were infected with T. annulata. The labeling obtained with the monoclonal antibodies to T. annulata was in agreement with other reports [9,15,22,36,37]. The presence of high numbers of T cells in the lymph nodes also showed that such cells were proliferating significantly, but not infiltrating classical target organs such as the spleen and liver as observed in cases of lymphoma [9,40].
Further confirmation of the aetiology of the disease was obtained by derivation of the gene sequence of the hypervariable region 4 of the parasite small subunit 18S ribosomal RNA, derived from cell lines established from two calves that were located on independent farms and sampled 14 months apart. Both sequences showed 100% identity with the sequence determined for the Ankara strain of T. annulata. Indeed, the sequence is 99-100% identical to all the 18s rRNA sequences of T. annulata deposited in the databases, including those of isolates from countries that border Portugal (Spain and France) and from more distant endemic geographical regions (Turkey, Tunisia, India and China). A significant alignment to the sequence derived for T. lestoquardi of sheep (99% identity) was also obtained, while sequences derived for all other species gave 97% or less identity. Therefore, based on the 18S rRNA gene identity, discrimination between T. lestoquardi and T. annulata may be problematic, particularly when analysing samples from Hyalomma tick vectors or sheep coinfected with both species [38]. It should be noted, however, that T. lestoquardi is unable to infect cattle [20] and, therefore, cannot be the aetiology of the pathological findings described in this study.
Based on the above results we conclude that the cases of acute disease identified in Portugal were caused by infection of the calves by T. annulata that generated a high level of morbidity and mortality. Since such cases have never been previously reported in Portugal, it is possible that more virulent strains of the parasite have established in the country, or epidemiological circumstances have altered to allow increased tick/parasite challenge or host susceptibility. Given the potential threat of this disease to livestock production in Portugal, a more detailed epidemiological survey is warranted.
The complexity of the T. annulata biological cycle has hindered complete understanding of the pathogenesis of tropical theileriosis, including how lesions found in a range of tissues and organs of affected animals are generated. Forsyth and others [15] postulated that the pathological changes observed in different organs and tissues are the result of proliferation of infected macrophages, while other clinical symptoms result from pro-inflammatory cytokines produced by these cells, mainly TNF-α [26]. The results of our study, however, do not indicate that tissue lesions are solely caused by proliferation of the infected cell as previously proposed [7,9,11,25]. Thus, phenotypic characterization of cells for all the microscopic lesions observed indicated that they contained mature uninfected T lymphoid cells and macrophages. The presence of these cell types in the lesions could be induced by generation of the immune response against T. annulata infection [7,9,11,18], and the tissue lesions associated with acute tropical theileriosis may result from damage caused by the host immune response to different phases of infection [28,29]. For example, macroschizont-infected host macrophages produce proinflamatory cytokines (IL-1α, IL-1β, IL-6, IL-10, TNF-α and INF-α) that may enhance initial establishment of the infection and cause disease [7,10,11,15,19,26], while metastasis of infected cells throughout tissues of the body is thought to involve the expression of metalloproteinases [1,2]. Infected macrophages also have the ability to stimulate an aberrant non-specific proliferation of naive T-cells leading to the production of high levels of INF-γ and IL-2, which can stimulate infected and non-infected macrophages to proliferate, but block an efficient specific immune response [3,11,15,18]. Such a disruption of the immune response is likely to result in disease pathology. A more complete understanding of how T. annulata manipulates the immune response will require detailed knowledge of the molecular mechanisms Theileria parasites utilize to activate and control the gene expression profile of the infected leukocytes [18,35].
Figures and Tables
Fig. 1
(A) Blood smear showing three ring forms of Theileria annulata piroplasms in red blood cells. (B) Lymph node smear showing a schizont in a mononuclear cell. Giemsa stain, ×1,000.
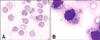
Fig. 2
(A) Widespread hemorrhagic nodules in subcutaneous tissue and abdominal muscles of calf (animal No.7). (B) Pale and hemorrhagic nodules are inserted in the tongue and the laryngeal and pharyngeal mucosa of calf (animal No.4).

Fig. 3
Lymphoid neoplastic-like cells (A) in a nodule infiltrating the skeletal muscle (H&E stain, ×100), with indistinct cell-membrane, high nuclear/cytoplasmic ratio and occasionally indented nuclei (B) (H&E stain, ×400). (C) Cells positive to CD3 (rabbit anti-CD3, streptavidin-biotin-peroxidase, Mayer's hematoxylin counterstain, ×100; Inset ×400). (D) Cells positive to MAC387 (mouse anti-human macrophages, streptavidin-biotin-peroxidase, Mayer's hematoxylin counterstain, ×100; Inset ×400).
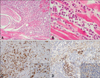
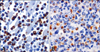
Fig. 4
Staining of Theileria annulata infected cells with monoclonal antibodies 1C7 (A) and 1C12 (B). Streptavidin-biotin-peroxidase, Mayer's hematoxylin counterstain, ×400.
Acknowledgments
The authors would like to thank Luísa Fialho, Maria Rosário Luís, Iolanda Fernandes and Dr. Rita Cardoso for technical assistance. This work was supported by Centro de Investigação Interdisciplinar em Sanidade Animal (CIISA).
References
1. Adamson R, Hall R. A role for matrix metalloproteinases in the pathology and attenuation of Theileria annulata infections. Parasitol Today. 1997. 13:390–393.

2. Adamson R, Logan M, Kinnaird J, Langsley G, Hall R. Loss of matrix metalloproteinase 9 activity in Theileria annulata-attenuated cells is at the transcriptional level and is associated with differentially expressed AP-1 species. Mol Biochem Parasitol. 2000. 106:51–61.

3. Ahmed JS, Schnittger L, Mehlhorn H. Review: Theileria schizonts induce fundamental alterations in their host cells. Parasitol Res. 1999. 85:527–538.

4. Biermann R, Schnittger L, Beyer D, Ahmed JS. Initiation of translation and cellular localization of Theileria annulata casein kinase IIα: implication for its role in host cell transformation. J Cell Physiol. 2003. 196:444–453.

5. Brown CGD. Control of Tropical Theileriosis (Theileria annulata infection) of cattle. Parassitologia. 1990. 32:23–31.
6. Brown CGD. Dynamics and impact of tick-borne diseases of cattle. Trop Anim Health Prod. 1997. 29:1S–3S.

7. Brown DJ, Campbell JD, Russell GC, Hopkins J, Glass EJ. T cell activation by Theileria annulata-infected macrophages correlates with cytokine production. Clin Exp Immunol. 1995. 102:507–514.

8. Campbell JD, Brown DJ, Glass EJ, Hall FR, Spooner RL. Theileria annulata sporozoite targets. Parasite Immunol. 1994. 16:501–505.
9. Campbell JD, Howie SE, Odling KA, Glass EJ. Theileria annulata induces abberrant T cell activation in vitro and in vivo. Clin Exp Immunol. 1995. 99:203–210.

10. Campbell JDM, Spooner RL. Macrophages behaving badly: infected cells and subversion of immune responses to Theileria annulata. Parasitol Today. 1999. 15:10–16.
11. Collins RA, Sopp P, Gelder KI, Morrison WI, Howard CJ. Bovine gamma/delta TcR+ T lymphocytes are stimulated to proliferate by autologous Theileria annulata-infected cells in the presence of interleukin-2. Scand J Immunol. 1996. 44:444–452.

12. DeMartini JC, Moulton JE. Responses of the bovine lymphatic system to infection by Theileria parva. I. Histology and ultrastructure of lymph nodes in experimentally-infected calves. J Comp Pathol. 1973. 83:281–298.

13. Dobbelaere D, Heussler V. Transformation of leukocytes by Theileria parva and T. annulata. Annu Rev Microbiol. 1999. 53:1–42.
14. D'Oliveira C, van der Weide M, Habela MA, Jacquiet P, Jongejan F. Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol. 1995. 33:2665–2669.
15. Forsyth LMG, Minns FC, Kirvar E, Adamson RE, Hall FR, McOrist S, Brown CGD, Preston PM. Tissue damage in cattle infected with Theileria annulata accompanied by metastasis of cytokine-producing, schizont-infected mononuclear phagocytes. J Comp Pathol. 1999. 120:39–57.

16. Gill BS, Bhattacharyulu Y, Kaur D. Symptoms and Pathology of experimental bovine tropical theileriosis (Theileria annulata infection). Ann Parasitol Hum Comp. 1977. 52:597–608.

17. Glascodine J, Tetley L, Tait A, Brown D, Shiels B. Developmental expression of a Theileria annulata merozoite surface antigen. Mol Biochem Parasitol. 1990. 40:105–112.

18. Glass EJ. The balance between protective immunity and pathogenesis in tropical theileriosis: what we need to know to design effective vaccines for the future. Res Vet Sci. 2001. 70:71–75.

19. Graham SP, Brown DJ, Vatansever Z, Waddington D, Taylor LH, Nichani AK, Campbell JDM, Adamson RE, Glass EJ, Spooner RL. Proinflammatory cytokine expression by Theileria annulata infected cell lines correlates with the pathology they cause in vivo. Vaccine. 2001. 19:2932–2944.

20. Leemans I, Brown D, Hooshmand-Rad P, Kirvar E, Uggla A. Infectivity and cross-immunity studies of Theileria lestoquardi and Theileria annulata in sheep and cattle: I. In vivo responses. Vet Parasitol. 1999. 82:179–192.

21. Leitão JLS. Teileriose bovina em Portugal. Repositório de Trabalhos do Laboratório Central de Patologia Veterinária. 1945. II:1–8.
22. Manuja A, Malhotra DV, Sikka VK, Sangwan AK, Sharma R, Kumar B, Mehta BD, Gulati BR, Nichani AK. Isolates of Theileria annulata collected from different parts of India show phenotypic and genetic diversity. Vet Parasitol. 2006. 137:242–252.

23. Mehlhorn H, Schein E. The piroplasms: life cycle and sexual stages. Adv Parasitol. 1984. 23:37–103.

24. Moreau M, Thibaud J, Miled LB, Chaussepied M, Baumgartner M, Davis WC, Minoprio P, Langsley G. Theileria annulata in CD5+ macrophages and B1 B cells. Infect Immun. 1999. 67:6678–6682.

25. Nichani AK, Thorp BH, Brown CGD, Campbell JDM, Brown DJ, Ritchie M, Spooner RL. In vivo development of Theileria annulata: major changes in efferent lymph following infection with sporozoites or allogeneic schizont-infected mononuclear cells. Parasitology. 1999. 118:327–333.

26. Preston PM, Brown CGD, Entrican G, Richardson W, Boid R. Synthesis of tumour necrosis factor-alpha and interferons by mononuclear cells from Theileria annulata-infected cattle. Parasite Immunol. 1993. 15:525–534.

27. Preston PM, Brown CG, Bell-Sakyi L, Richardson W, Sanderson A. Tropical theileriosis in Bos taurus and Bos taurus cross Bos indicus calves: response to infection with graded doses of sporozoites of Theileria annulata. Res Vet Sci. 1992. 53:230–243.

28. Preston PM, Hall FR, Glass EJ, Campbell JDM, Darghouth MA, Ahmed JS, Shiels BR, Spooner RL, Jongejan F, Brown CGD. Innate and adaptive immune responses co-operate to protect cattle against Theileria annulata. Parasitol Today. 1999. 15:268–274.

29. Preston PM, Jongejan F. Protective immune mechanisms to ticks and tick-borne diseases of ruminants. Parasitol Today. 1999. 15:255–258.

30. Renneker S, Kullmann B, Gerber S, Dobschanski J, Bakheit MA, Geysen D, Shiels B, Tait A, Ahmed JS, Seitzer U. Development of a competitive ELISA for detection of Theileria annulata infection. Transbound Emerg Dis. 2008. 55:249–256.

31. Rozen S, Skaletsky H. Misener S, Krawetz SA, editors. Primer3 on the WWW for general users and for biologist programmers. Bioinformatics Methods and Protocols. 2000. Totowa: Humana Press;365–386.

32. Sager H, Bertoni G, Jungi TW. Differences between B cell and macrophage transformation by the bovine parasite, Theileria annulata: a clonal approach. J Immunol. 1998. 161:335–341.
33. Sager H, Davis WC, Dobbelaere DA, Jungi TW. Macrophage-parasite relationship in theileriosis. Reversible phenotypic and functional dedifferentiation of macrophages infected with Theileria annulata. J Leukoc Biol. 1997. 61:459–468.

34. Sharma G, Sharma RD, Nichani AK. Successful long-term in vitro cultivation of Theileria annulata schizonts in media supplemented with homologous and heterologous sera. Vet Parasitol. 1998. 79:135–141.

35. Shiels B, Langsley G, Weir W, Pain A, McKellar S, Dobbelaere D. Alteration of host cell phenotype by Theileria annulata and Theileria parva: mining for manipulators in the parasite genomes. Int J Parasitol. 2006. 36:9–21.

36. Shiels B, McDougall C, Tait A, Brown CGD. Antigenic diversity of Theileria annulata macroschizonts. Vet Parasitol. 1986. 21:1–10.
37. Shiels BR, McDougall C, Tait A, Brown CGD. Identification of Infection-associated antigens in Theileria annulata transformed cells. Parasite Immunol. 1986. 8:69–77.

38. Spitalska E, Torina A, Cannella V, Caracappa S, Sparagano OA. Discrimination between Theileria lestoquardi and Theileria annulata in their vectors and hosts by RFLP based on the 18S rRNA gene. Parasitol Res. 2004. 94:318–320.

39. Spooner RL, Innes EA, Glass EJ, Brown CGD. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology. 1989. 66:284–288.
40. Valli VEO. Maxie MG, editor. The hematopoietic system. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. 2007. Vol. 2:5th ed. Philadelphia: Saunders;199–201.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download