Abstract
Outer membrane proteins of Pasteurella (P.) multocida have been known to be protective immunogens. Pasteurella lipoprotein E (PlpE) has been reported to be an important cross reactive outer membrane protein in P. multocida. The gene encoding the PlpE of P. multocida serotypes A: 3, B: 2 and D: 1 was amplified from the genomic DNA. The amplified products were cloned and the nucleotide sequence was determined. Sequence analysis of the recombinant clones revealed a single open reading frame of 1,011 bp, 1,008 bp and 1,017 bp encoding a protein with a calculated molecular mass of 37.829 kDa, 37.389 kDa and 37.965 kDa for serotypes A: 3, B: 2 and D: 1 respectively. The comparison of the plpE sequence in different capsular types revealed a high degree (>90%) of homology. Furthermore, the plpE gene of Haemorhhagic septicaemia causing serotype (B: 2) was expressed in E. coli and recombinant PlpE was strongly immunostained by antiserum against whole cell antigen, indicating that the protein is expressed in vivo.
The population of cattle and buffaloes in India is the largest in the world and represents a large portion of the Indian livestock industry. Hemorrhagic septicaemia (HS) is an economically important disease responsible for a high morbidity and mortality in cattle and buffaloes in India and other parts of Asia and Africa [8]. In India alone, it is estimated to cause economic losses of more than 10 million rupees annually [23]. HS is primarily caused by serotypes B: 2 and E: 2 of Pasteurella (P.) multocida. Besides B: 2, several other serotypes are associated with sporadic outbreaks of disease, particularly in feral ruminants [12]. Apart from HS causing serotype B: 2, serotype A has also been associated with HS like conditions in ruminants [14]. The organism is also responsible for bovine pneumonia, porcine pneumonia, fowl cholera, and snuffles in livestock and poultry [22].
Considering its significance as an important animal pathogen, research has been focused mainly on identifying the immunogens of P. multocida that can elicit cross-protective immunity and thus be used in vaccines against Pasteurellosis. Membrane antigens have been identified as potential immunogens and shown to elicit protective immune responses. It has been recognized that in vivo-grown P. multocida and membrane-associated proteins of in vivo grown cells can induce cross-protective immunity [10]. Partially purified membrane proteins, prepared from in vivo grown cells with apparent molecular weight of 39 kDa and 59 to 65 kDa have been reported to be associated with cross-protective immunity [20].
Outer membrane protein (OMP) profiling and western blot analysis have shown several immunogenic proteins in the outer membrane fractions of P. multocida [3,7,13,15]. Among these, a 39 kDa protein has been reported to be highly immunogenic and cross protective, when challenged with heterologous strains [11,21]. The monoclonal antibodies to this protein were found to be protective in mice against lethal challenge with virulent strains [2]. Later, Wu et al. [25] recognized 39 kDa cross protective immunogen as Pasteurella lipoprotein E (PlpE). They also reported the protective nature of recombinant PlpE (r-PlpE) against a challenge infection with virulent P. multocida strain X-73 serotype A: 1, in mice and chickens. PlpE has also been reported in other bacterial species [19].
Confer et al. [5] vaccinated cattle with recombinant PlpE of Mannheimia haemolytica which resulted in reduced severity of lung lesions in experimental study with virulent M. haemolytica. The plpE gene of P. multocida Pm70 encodes a lipoprotein of 335 amino acids and has 24.3% sequence homology with the plpE gene of M. haemolytica [17]. Nevertheless the major epitopes of the PlpEs of M. haemolytica and P. multocida Pm70 share no homology.
In this study, we described the molecular cloning and characterization of the plpE gene of P. multocida capsular type A: 3, B: 2 and D: 1 in order to determine the heterogeneity among these. Additionally, prokaryotic expression and purification of PlpE was carried out for immunological study.
Three isolates viz. P. multocida serotypes A: 3, B: 2 (vaccine strain P52) and D: 1 maintained in the Division of Bacteriology and Mycology, Indian Veterinary Research Institute, were employed as sources of the plpE gene. Escherichia coli (strain JM109) used as host for molecular cloning and expression of plpE gene, was grown in either Luria Bertani broth (Himedia Laboratories, India) at 37℃ or Luria Bertani agar containing ampicillin at a final concentration of 50 µg/mL.
The OMPs were extracted by the method described by Choi-Kim et al. [4]. Briefly the P. multocida serotype B: 2 (P-52) cells were grown in BHI broth (Himedia Laboratories, India), harvested and washed twice using sterile PBS (pH7.2). The cells were then suspended in 10 mM HEPES (pH 7.4) and disrupted by sonication, five jerks at 10 micron for 2 min each, at 30 sec intervals. The cell debris was removed by centrifugation at 1,700 × g for 20 min. The sonicated supernatant was then centrifuged at 100,000 × g at 4℃ for 60 min. The resultant Pellet was resuspended in 2 mL of 2% (W/V) sodium lauryl sarcosinate (Sigma, USA) in 10 mM HEPES (pH 7.4) and incubated at 22℃ for 60 min. The insoluble OMPs were sedimented by centrifugation at 100,000 × g at 4℃ for 60 min and the pellet was dissolved in PBS.
The whole cell method was employed to raise the hyper immune sera against P. multocida B: 2 [24]. The P. multocida B: 2 organisms were grown in casein sucrose yeast extract agar plate and the growth was harvested into PBS containing 0.3% formalin and turbidity was adjusted by Mac Farland's tube number 4. Rabbits were inoculated intravenously at 3~4 day intervals with 0.2, 0.5, 0.75, 1.0, 1.5 and finally 2.0 mL. One week after the final injection, a booster dose (0.5 mL of live culture) was given intravenously. The animals were bled after 10 days and the serum separated.
Genomic DNA of P. multocida serotypes A: 3, B: 2 and D: 1 were isolated using DNA isolation kit (Qiagen, USA). The plpE genes from all three serotypes were amplified by PCR using specific primers 5'-CCATGGGCATGAAACAAATCGTTTTAAA-3' (sense primer) and 5'-CTCGAGTTGTGCTTGGTGACTTTTTTC-3' (antisense primer). The PCR mixture consisting of 50 ng of template, 25 pmol of each of the primers, 200 µM of dNTPs mix, × 1 PCR buffer, 1.5 mM MgCl2 and 1 U of Taq DNA polymerase (MBI Fermentas, USA) were diluted to a 25 µL volume with milliQ water. PCR was performed with the initial denaturation at 94℃ for 10 min followed by 30 cycles of denaturation at 94℃ for 45 sec, annealing at 53℃ for 45 sec, extension at 72℃ for 1 min and final extension at 72℃ for 10 min. The amplified product was visualized by electrophoresis through 2% agarose gel (Bangalore Genei, India) prepared in × 1 Tris Acetate EDTA buffer and photographed.
The agarose gel containing DNA fragments was excised and the gel extraction of DNA fragments was carried out using Min Elute Gel extraction kit (Qiagen, USA). The Taq DNA polymerase-generated PCR products from each serotype were separately ligated to pDrive TA cloning vector (Qiagen, USA) and transformed into E. coli JM109 by rapid DNA ligation and a transformation kit (MBI Fermentas, USA). Preliminary identification of recombinant clones was done by blue/white screening. Plasmid isolation was carried out using Plasmid miniprep kit (Quiagen, USA) and the clones were confirmed by restriction digestion using NcoI and XhoI restriction endonuclease (MBI Fermentas, USA). The confirmed clones were then used for sequencing of the inserted gene. The obtained sequences were compared with the reported plpE gene of other P. multocida serotypes viz. X-73 (A: 1), P-470 (A: 3), P-1059 (A: 3), P-1662 (A: 4), P-61 (D: 3) and ATCC 12948 (D: 11), using DNAStar software (DNASTAR, USA). Secondary structure predictions and physiochemical properties of PlpE in the form of the algorithm were generated with the Protean program (DNAStar, USA).
The truncated form of plpE gene without the sequence encoding the putative signal peptide was amplified using genomic DNA of P. multocida serotype B: 2. The oligonucleotide sequence for the forward primer was 5'-CCATGGCATGTAGCGGTGGTGGC-3' starting 58 nucleotides downstream of the 5' end and 5'-CTCGAGTTATTGTGCTTGGTGAC-3' for reverse primer. The 960 bp amplimer thus obtained was digested with restriction enzymes NcoI and XhoI before cloning into expression vector pPRO.EX.HTb, which was double digested with the same enzyme pair. The digested fragments were ligated followed by transformation into competent E. coli JM109. Subsequently the transformed cells were plated on LB agar containing ampicillin (50 µg/mL). The recombinant plasmids were identified by restriction endonuclease analysis. For the synthesis of recombinant protein, a single colony of E. coli JM109 harboring the plpE gene in pPRO.EX.HTb was inoculated into 100 mL of LB broth supplemented with ampicillin and incubated with shaking at 37℃ until the culture reached an OD of 0.4 to 0.6 at A600 nm (~4 h incubation). Mid log phase culture was induced by 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma, USA) and 1 mM MgCl2. The induced culture was incubated at 30℃ for 6 h with vigorous shaking (300 rpm).
The culture was harvested and lysed by using lysis buffer (100 mM NaH2PO4, 10 mM Tris, 8 M urea, pH 8.0). The bacterial lysate was centrifuged at 10,000 × g for 30 min at 4℃ to remove insoluble cellular debris. The supernatant containing the recombinant protein was loaded onto affinity column packed with nickel chelating resin (Qiagen, USA) that selectively binds recombinant proteins with 6 histadine residues (His-Tag). The lysate was washed with 10 ml of wash buffer (100 mM NaH2PO4, 10 mM Tris, 8 M urea, pH 6.3) for removing the non-specific proteins. The recombinant protein bound to resin was then eluted with a low pH buffer.
Samples were analyzed on a polyacrylamide gel using gels of 12% acrylamide contents and 3.3% cross linking. The bacterial OMP preparation and purified recombinant PlpE were applied to 12% gels and electrophoresed at 20mA. Before loading, samples were boiled for 3.5 min in an equal volume of buffer containing 2% SDS. Samples run under reducing conditions were boiled in the presence of 5% 2-mercaptoethanol. The gels were stained with Coomassie blue R-250 (Bio-Rad, USA) for detection of proteins. For Western blots, the proteins were transferred to Nitrocellulose membranes at 80 V for 2 h. The membranes were placed in 5 mL blocking buffer (5% Skim milk powder in TBS) and incubated at 4℃ overnight followed by washing twice with TBS-T [1 M Tris HCl (pH 8.0), 5 M NaCl, and Tween 20 (0.05%)] for 10 min each. Subsequently, the membranes were probed with hyper immune sera raised against P. multocida serotype B: 2 diluted in blocking buffer (1 : 500) and washed. Rabbit anti-goat peroxidase-conjugate (diluted 1 : 5,000) (Sigma, USA) was used as secondary antibody. The blots were developed by adding chromogenic substrate solution (Bio-Rad, USA) for 5-10 min. The reaction was terminated by washing the membrane with distilled water.
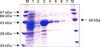
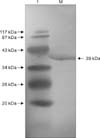
OMPs of P. multocida B: 2 were extracted and upon sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) separation, bands of apparent molecular weight 16, 27, 29, 32, 39 and 47 kDa proteins were observed in SDS-PAGE (Fig. 1). The polypeptides of approximate molecular weight 32 and 39 kDa were found to be major OMPs. On Western blots, 32 and 39 kDa proteins were identified as major immunodominant proteins, giving a strong immunostaining reaction with hyper-immune sera against whole cell antigen of P. multocida serotype B: 2 strain P52.
The plpE gene from P. multocida serotype A: 3, B: 2 and D: 1 was successfully amplified using the plpE specific primers. A single PCR product with a molecular size of approximately 1 kb was amplified in all of the three serotypes of P. multocida (Fig. 2). The amplified products were separately cloned into pDrive TA cloning vector (Qiagen, USA). The restriction digestion of each of the recombinant clones with NcoI and XhoI enzymes confirmed the presence of an approximately 1,010 bp sequence within the recombinant plasmid.
The sequence data for the plpE gene of P. multocida serotypes A: 3, B: 2 and D: 1 have been submitted to the GenBank database and assigned the accession numbers GQ202239, GQ202240 and GQ202241.
The nucleotide sequence of plpE revealed a putative open reading frame, beginning with an ATG initiation codon which remained open to the end of the sequence obtained. The 1kb functional plpE gene was found to be 1,011 bp, 1,008 bp and 1,017 bp in length for the P. multocida serotypes A: 3, B: 2 and D: 1, respectively.
The plpE gene of Indian isolates of P. multocida serotypes A: 3, B: 2 and D: 1 were aligned for homology with the reported sequence of P. multocida strains X-73 (A: 1), P-470 (A: 3), P-1059 (A: 3), P-1662 (A: 4), P-61 (D: 3), ATCC 12948 (D: 11) and the percentage of identity and divergence among nine strains of P. multocida has been depicted in Fig. 3. The result of phylogenetic tree analysis showed close relationship between Indian isolate of P. multocida serotype A: 3 to the reported strain X-73, while serotype D: 1 was close to strain ATCC 12948. However, serotype B: 2 of P. multocida was found to be distantly related to the X-73 and ATCC 12948 strains of P. multocida. The detailed description of the isolates used in this study for plpE gene sequence comparison has been depicted in Table 1.
The deduced sequences of PlpEs of P. multocida serotypes A: 3, B: 2, and D: 1 were found to contain 336, 335 and 338 amino acids, respectively, having predicted molecular masses of 37.829, 37.389 and 37.965 kDa, respectively. When the amino acid sequences were compared it was observed that two amino acids (both serine) were found missing at the 26th and 27th position in the P. multocida serotype A: 3 when compared to serotype D: 1. Additionally, methionine was found missing at the 59th position in serotype B: 2.
The physiochemical analysis of PlpE using Protean software showed a typical hydrophobic stretch at the N terminus, which corresponded to the putative signal peptide. The protein sequences following signal peptide had predominant hydrophilic regions, with smaller intervening hydrophobic stretches throughout the protein. The predicted protein had predominant antigenic indices throughout the length and region with probable antigenic determinant of protein was observed between 1-28 amino acids (Fig. 4).
The ~960 bp plpE gene product without signal peptide sequence was amplified from P. multocida genome and cloned directionally in expression vector pPRO.EX.HTb. The recombinant E. coli Jm109 cells were induced by IPTG and the expressed plpE fused with polyhistadine tag was purified by nickel chelating affinity chromatography. SDS-PAGE electrophoresis of the eluted fraction showed a very intense protein band of ~39 kDa (Fig. 5). Western blot analysis using homologous hyper immune serum against whole cell antigen further confirmed the antigenicity of 39 kDa recombinant PlpE (Fig. 6).
In this study, we characterized the plpE gene of P. multocida serotypes A, B and D followed by expression and immunoblot of the PlpE of P. multocida P52 (the vaccine strain used in India). OMPs have been found to be potent immunogens and protective to mice, rabbits, chicken and calves [6,16,26]. A 39 kDa cross-protective antigen was identified by Rimler [20] in P. multocida A: 3 (strain P1059) which induced active and passive cross-protection in chickens and turkeys [11,21]. The monoclonal antibodies against 39 kDa protein of P. multocida P-1059 (serovar A: 3) were produced and characterized by Ali et al. [1,2]. Mice passively immunized with the monoclonal antibodies to 39 kDa protein were found resistant to lethal challenge with virulent strains. In 2007, Wu et al. [25] observed that mice immunized with purified recombinant PlpE (r-PlpE) was resistant to homologous and heterologous challenge with P. multocida. Also, the r-PlpE was found to confer good protection against challenge infection with P. multocida strain X-73 (A: 1) in chickens.
The plpE genes of A: 3, B: 2 and D: 1 serotypes of P. multocida were amplified using the primers designed on the sequence of the plpE gene of P. multocida A: 3 which was approximately 1kb in all the three isolates. Upon comparison with reported sequences of plpE gene of P. multocida strains X-73 (A: 1), P-470 (A: 3), P-1059 (A: 3), P-1662 (A: 4), P-61 (D: 3), ATCC 12948 (D: 11), more than 90% homology was observed with Indian isolates of P. multocida serotypes A: 3. B: 2 and D: 1. The result showed the presence of plpE gene in all three serotypes of Indian isolates. The nucleotide sequence of HS causing P. multocida serotype B: 2 had 92.8% and 94.4% identity with other Indian isolates of P. multocida serotypes A: 3 and D: 1 respectively. The finding indicated that Indian isolates of P. multocida are closely related to each other and also to other strains on the basis of the plpE gene sequence. Wu et al. [25], also observed 90.8-100% sequence homology of the plpE gene, shared by different strains of P. multocida serotypes.
The nucleotide sequences of the Indian isolates of P. multocida serotypes A: 3, B: 2 and D: 1 were converted to amino acids and compared for homology. The amino acid sequence showed variation in composition among serotypes A: 3, B: 2 and D: 1. The absence of one or more amino acids may attribute to the difference in immunogenicity of the plpE among these serotypes; this can be confirmed by detailed proteomic analysis of the recombinant protein.
The predominant hydrophilic regions were found dispersed throughout the length of protein. The above finding was further supported by surface probability assay of the PlpE, by which it could be inferred that the PlpE has putative surface exposed regions randomly distributed throughout the protein. Similar findings have been reported in other PlpE of M. haemolytica and OmlA of A. pleuropneumoniae by Gerlach et al. [9] and Pandher et al. [19].
The PlpE was expressed in E. coli JM109 and the apparent molecular mass of the expressed PlpE was found to be ~39 kDa when analyzed by SDS-PAGE. Western Blot analysis revealed the presence of a single ~39 kDa immunogenic band, indicating the antigenic and immunodominant nature of the PlpE. This statement was further supported by the SDS-PAGE and immunoblot analysis of OMPs of P. multocida serotype B: 2 (P52). The OMPs were extracted and separated by SDS-PAGE, which revealed presence of major outer membrane fractions with molecular masses of approximately 39 kDa. Immunoblot of OMPs probed with hyperimmune sera against whole cell antigen of P. multocida P52 revealed a deeply immunostained band corresponding to a molecular weight of 39 kDa. The results clearly indicated that PlpE is most likely to be a 39 kDa cross protective immunodominant antigen, as described by Ibrahim et al. [11] and Rimler [21]. It also indicates a satisfactory level of expression of PlpE grown in in-vitro conditions.
The findings of the present study revealed that PlpE is present in all three Indian isolates of P. multocida serotypes A: 3, B: 2 and D: 1. The antigenic uniformity in this protein and extent of amino acid similarity supports its cross protective nature among the three serotypes. The western blot analysis further confirmed that the PlpE is one of the major immunodominant proteins in P. multocida.
Figures and Tables
Fig. 1
SDS-PAGE of outer membrane preparation. Lane M represents protein molecular weight marker. The most prominent band corresponding to molecular weights of 39 kDa and 32 kDa is indicated.
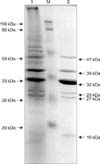
Fig. 2
The plpE gene amplified by PCR using genomic DNA of P. multocida. Lane M represents DNA ladder and Lane 1-3 shows an amplified plpE gene in P. multocida serotypes A: 3, B: 2 and D: 3 respectively.
Fig. 3
DNA identity matrix based on pair wise comparison of plpE sequences of different strains of Pasteurella multocida.

Fig. 4
Hydrophilicity, antigenicity and surface probability plot of deduced amino acid sequence of PlpE of P. multocida strain P-52 (serotype B: 2). Positive values represent hydrophilic, antigenic and surface exposed regions.

Fig. 5
Expression and purification of r-PlpE in E. coli JM109. Lane M represents protein molecular weight marker, Lane 1 contains lysis buffer follow through, Lane 2 contains wash buffer follow through and Lanes 3-7 contains first, second, third, fourth and fifth eluted fractions.
References
1. Ali HA, Sawada T, Hatakeyama H, Ohtsuki N, Itoh O. Characterization of a 39 kDa capsular protein of avian Pasteurella multocida using monoclonal antibodies. Vet Microbiol. 2004. 100:43–53.

2. Ali HA, Sawada T, Noda K. Protectivity of an immunoaffinity-purified 39 kDa capsular protein of avian Pasteurella multocida in mice. J Vet Med Sci. 2004. 66:1603–1604.

3. Ataei S, Burchmore R, Christopher Hodgson J, Finucane A, Parton R, Coote JG. Identification of immunogenic proteins associated with protection against haemorrhagic septicaemia after vaccination of calves with a live-attenuated aroA derivative of Pasteurella multocida B: 2. Res Vet Sci. 2009. 87:207–210.

4. Choi-Kim K, Maheswaran SK, Felice LJ, Molitor TW. Relationship between the iron regulated outer membrane proteins and the outer membrane proteins of in vivo grown Pasteurella multocida. Vet Microbiol. 1991. 28:75–92.

5. Confer AW, Ayalew S, Panciera RJ, Montelongo M, Whitworth LC, Hammer JD. Immunogenicity of recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE and augmentation of a commercial vaccine. Vaccine. 2003. 21:2821–2829.

6. Confer AW, Ayalew S, Panciera RJ, Montelongo M, Wray JH. Recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE enhances commercial M. haemolytica vaccine induced resistance against serotype 6 challenge. Vaccine. 2006. 24:2248–2255.

7. Dey S, Singh VP, Kumar AA, Srivastava SK, Singh N. Molecular identification of Indian isolates of Pasteurella multocida serotype B: 2 by different PCR assay. Indian J Anim Sci. 2007. 77:317–319.
8. Dutta J, Rathore BS, Mullick SG, Singh R, Sharma GC. Epidemiological studies and occurrence of haemorrhagic septicaemia in India. Indian Vet J. 1990. 67:893–899.
9. Gerlach GF, Anderson C, Klashinsky S, Rossi-Campos A, Potter AA, Willson PJ. Molecular characterization of a protective outer membrane lipoprotein (OmlA) from Actinobacillus pleuropneumoniae serotype 1. Infect Immun. 1993. 61:565–572.

10. Heddleston KL, Rebers PA. Fowl cholera: cross-immunity induced in turkeys with formalin killed in-vivo-propagated Pasteurella multocida. Avian Dis. 1972. 16:578–586.

11. Ibrahim RS, Sawada T, Shahata M, Ibrahim A. Cross-protection and antigen expression by chicken embryo-grown Pasteurella multocida in chickens. J Comp Pathol. 2000. 123:278–284.

12. Jones TO, Hussaini SN. Outbreaks of Pasteurella multocida septicaemia in fallow deer (Dama dama). Vet Rec. 1982. 110:451–452.

13. Kedrak A, Borkowska-Opacka B. Immunological response to outer membrane proteins of Pasteurella multocida serotype A: 3 in calves. Bull Vet Inst Pulawy. 2003. 47:387–394.
14. Kumar AA, Shivachandra SB, Biswas A, Singh VP, Singh VP, Srivastava SK. Prevalent serotypes of Pasteurella multocida isolated from different animal and avian species in India. Vet Res Commun. 2004. 28:657–667.

15. Lu YS, Afendis SJ, Pakes SP. Identification of immunogenic outer membrane proteins of Pasteurella multocida 3:A in rabbits. Infect Immun. 1988. 56:1532–1537.

16. Lu YS, Lai WC, Pakes SP, Stefanu C. The outer membrane of Pasteurella multocida 3:A protects rabbits against homologous challenge. Infect Immun. 1991. 59:4517–4523.

17. May BJ, Zhang Q, Li LL, Paustian ML, Whittam TS, Kapur V. Complete genomic sequence of Pasteurella multocida, Pm70. Proc Natl Acad Sci USA. 2001. 98:3460–3465.
18. Pal A, Srivastava SK, Singh VP. Heat modifiability of outer membrane protein of Pasteurella multocida serotype B: 2. Indian J Exp Biol. 2002. 40:106–108.
19. Pandher K, Confer AW, Murphy GL. Genetic and immunologic analyses of PlpE, a lipoprotein important in complement-mediated killing of Pasteurella haemolytica serotype 1. Infect Immun. 1998. 66:5613–5619.

20. Rimler RB. Partial purification of cross-protection factor(s) from Pasteurella multocida. Avian Dis. 1994. 38:778–789.

21. Rimler RB. Purification of a cross-protective antigen from Pasteurella multocida grown in vitro and in vivo. Avian Dis. 2001. 45:572–580.

22. Rimler RB, Rhoades KR. Adlam C, Rutter JM, editors. Pasteurella multocida. Pasteurella and Pasteurellosis. 1989. London: Academic Press;34–67.
23. Venkataramanan R, Bandyopadhyay SK, Oberoi MS. Present status and strategies for the control of transboundary and orher economically important animal diseases in India: A review. Indian J Anim Sci. 2005. 75:456–464.
24. Wijewardana TG. Haemorrhagic Septicemia: Diagnostic and Vaccine Production Procedures. 1992. Peradenya: Veterinary Research Institute;9–10.
25. Wu JR, Shien JH, Shieh HK, Chen CF, Chang PC. Protective immunity conferred by recombinant Pasteurella multocida lipoprotein E (PlpE). Vaccine. 2007. 25:4140–4148.

26. Zhang H, Ainsworth AJ, Montgomery RD. Use of a 35.5 kDa cell membrane composition of Pasteurella multocida and an anti-idiotype antibody to induce protective immunity in leghorn chickens. Vet Immunol Immunopathol. 1994. 41:89–100.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download