Abstract
The aim of this study was to evaluate the presence of Helicobacter (H.) spp. in swine affected by gastric ulceration. Stomachs from 400 regularly slaughtered swine were subjected to gross pathological examination to evaluate the presence of gastric ulcers. Sixty-five samples collected from ulcerated pars esophagea and 15 samples from non-ulcerated pyloric portions were submitted to histopathological and molecular analyses, to detect Helicobacter spp., H. suis and H. pylori by PCR. Feces and saliva swabs were also collected from 25 animals in order to detect in vivo the presence of Helicobacter spp.. Gastric ulcers were detected in 373 cases (93%). The presence of ulcers in association with inflammatory processes was further confirmed by histological examination. Forty-nine percent (32/65) of the ulcerated esophageal portions as well as 53% (8/15) of the non-ulcerated pyloric portions were positive for Helicobacter spp. by PCR. The Helicobacter spp. positive samples were also positive for H. suis, while H. pylori was not detected. These results were confirmed by restriction enzyme analysis. With regard to feces and saliva samples, 15/25 (60%) and 16/25 (64%) were positive for Helicobacter spp. PCR, respectively but all were negative in H. suis and H. pylori specific PCR.
Ulceration in the non-glandular part of the stomach of pigs has been reported in several countries [12,30,31]. It is a disease of complex etiology in which multiple factors are involved including dietary and stress conditions [19] such as small particle sizes of feed, interruption of feed intake and the presence of highly fermentable carbohydrates [1]. Its etiology also seems to be associated with various infectious diseases such as porcine reproductive respiratory syndrome (PRRS), post-weaning multisystemic wasting syndrome (PMWS) and porcine dermatitis nephrophathy syndrome (PDNS) [14,32]. Ulcers of the non-glandular part of the stomach have been associated with Helicobacter (H.) suis [4,5,28,31], but the exact role of H. suis in porcine gastric pathology is a subject of considerable debate. Indeed, several authors [11,23,26,33] did not find this association while it has been recently reported that gastric ulcers of the non-glandular part of the stomach were induced in pigs experimentally infected with H. suis [13]. In any case, it was demonstrated that H. suis causes gastritis in experimentally and naturally infected pigs [3,11,18,24,26,28]. H. suis is the main Helicobacter species colonizing the stomachs of pigs and is the most prevalent gastric non-H. pylori Helicobacter species in humans [35]. There are strong indications that pigs may be a source of H. suis infection in humans, while animals may be occasionally infected with H. pylori [7,9,34]; Krakowka et al. [20] demonstrated the occurrence of gastric ulcers in gnotobiotic piglets experimentally infected with H. pylori. Our study was conducted to evaluate the presence of Helicobacter spp., H. suis and H. pylori by PCR in swine affected by gastric ulcers. Moreover, the presence of Helicobacter spp. was investigated in feces and saliva samples in order to assess the feasibility of an in vivo detection method.
A total of 400 stomachs collected at slaughter from 10-month-old pigs (50% males and 50% females) coming from two intensive closed-cycle-based breeding farms in the centre of Italy, were examined to evaluate the presence of gastric ulcers. For molecular studies, feces and saliva swabs were also collected from 25 subjects belonging to the same groups and stored at -80℃ until use. Each stomach was individually examined using disposable blades and gloves to avoid cross contamination. The stomachs were opened longitudinally along the greater curvature. The gastric content was gently removed and the mucosa was examined to evaluate the presence of pathological changes. Sixty-five samples from the pars esophagea were taken from pigs with ulcers and 15 samples were collected from pyloric portions without macroscopic signs of ulcers. All these samples were cut and divided into two parts and then stored in an ice box (4℃) for transport to the laboratory for histopathology and molecular analyses.
The samples were fixed in 10% phosphate-buffered formalin, dehydrated, embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin for light microscopic examination.
All the samples were stored at -80℃ until use. The DNA was extracted from all the samples using the Charge Switch gDNA mini tissue kit (Invitrogen, UK) in accordance with the manufacturer's protocol. For PCR analysis, specific primers (Invitrogen, UK) and PCR protocols were used (Table 1) and the genomic DNA of H. pylori (ATCC 43504) was employed as a positive control for Helicobacter spp. and H. pylori PCRs. Reactions were performed in a 50-µL volume consisting of 1.25 µg of extracted DNA, 2 U of Taq DNA polymerase, 0.5 µM of each primer, 1.5 mM (2.5 mM for H. suis PCR) of MgCl2, 200 µM of dNTPs mix and ×1 PCR buffer (Invitrogen, UK) in a Gene Amp PCR System 2400 thermal cycler (Applied Biosystems, USA), according to the specific PCRs conditions (Table 1). PCR products were resolved by electrophoresis in 1~1.5% agarose gel containing 4 µL of GelRed (nucleic acid gel stain, ×10,000 in water) per 100 mL (Biotium, USA) and examined by transillumination (Euroclone, Italy).
To support H. suis PCR analysis, 10-µL samples, of Helicobacter spp. PCR product were digested with 10 U of MboI (Gibco, UK) for 4 h at 37℃ [27]. The digested samples were analyzed by electrophoresis in 1.5% agarose gel and examined by transillumination.
Eight H. suis PCR products from the gastric samples were sequenced using V832f primer, specific to the H. suis 16S rRNA gene, and 8 Helicobacter spp. PCR products from feces and saliva samples were sequenced using H276f primer, specific to the 16S rRNA gene of Helicobacter genus. All samples for nucleotide sequencing were obtained by PCR product purification using the Jet Quick gel extraction spin kit (Genomed GmbH, Germany) in accordance with the manufacturer's protocol. PCR fragments and primers were premixed in the same tube, and sent to the company (Primm Srl, Italy), for sequencing. The DNA sequences obtained were aligned and compared with the BLAST database (NCBI, USA).
Three hundred and seventy-three of the 400 stomachs (93%) examined at the slaughterhouse showed ulcers across the small curvature of the pars esophagea, often associated with extensive hyperemic areas. At times, the edges of the ulcers appeared thickened and firm. Histological examinations frequently revealed lymphocytic and plasmacytic cellular infiltrates in the glandular part in acute evolution and lymphoid follicle formations in the gastric lamina propria; instead lymphofollicular gastritis was predominant in chronic ulcerous processes (Fig. 1). Proliferation of granulation tissue and hyperplasia of the epithelium were also visible near ulcers in sub acute and chronic evolution.
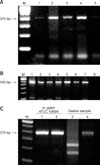
PCR analysis of the gastric samples revealed the presence of Helicobacter spp. in 30/65 (49%) of the esophago-gastric ulcer samples and 8/15 (53%) of the samples from pyloric mucosa without lesions. Moreover, all Helicobacter spp. positive samples were also positive for H. suis PCR (Figs. 2A and B). H. pylori was not detected in any samples. PCR analysis of feces and saliva samples showed that 15/25 (60%) and 16/25 (64%) were positive for Helicobacter spp., respectively. None of the samples was positive for H. suis or H. pylori.
As expected, MboI cleaved the 376 bp Helicobacter spp. PCR products, amplified from the gastric samples, into two fragments of 147 and 229 bp, with the exception of those from H. pylori (ATCC 43504) used as negative controls (Fig. 2C).
Sequence analysis from 8 gastric samples positive for H. suis PCR showed 99% homology with the 16S rRNA gene of H. suis [2]. Sequence analysis from 8 feces samples and from 8 saliva samples positive for Helicobacter spp. showed 99% homology with the 16S rRNA gene of Helicobacter genus.
Ulceration of the non-glandular esophageal portion of the stomach of feeder pigs, known as gastroesophageal ulceration, is a common and serious problem in swine production [10]. Its etiology is multifactorial, including genetic, nutritional, environmental and infectious causes; indeed, animals affected by PRRS, PMWS and PDNS usually show gastric lesions at necropsy, but the relationship seems to be circumstantial [14,19]. Our results showed that ulcers of the non-glandular esophageal portion of the stomach were present in a high percentage of the slaughtered pigs (93%), as confirmed by histological examination. Ulcers were not macroscopically observed in the glandular part (pyloric portion), while histological examination frequently showed lymphocytic and plasmacytic cellular infiltrates, as reported elsewhere [17]. PCR analysis of gastric samples from the pars esophagea (with ulcers) and from the pyloric portion (without ulcers) revealed the presence of Helicobacter spp. in 49% and 53% specimens respectively. Moyaert et al. [25] compared different Helicobacter spp. PCR primers showing that the ones described by Riley et al. [29] and used in this study are highly capable of correctly identifying Helicobacter strains and have a moderate ability to exclude non target species, giving amplicons of the correct size. To determine if the microorganisms present in the gastric samples were H. suis or H. pylori, the Helicobacter spp. positive samples were submitted to H. suis and H. pylori specific PCR. All the samples were positive for H. suis and negative for H. pylori. The results for H. suis were confirmed by restriction enzyme analysis and DNA sequencing. Our results may indicate that H. suis colonizes both the pars esophagea [31] and the pyloric portion with ulcer occurrence only in the pars esophagea. The presence of ulcers in the pars esophagea suggests a different susceptibility of the two anatomic regions to the same pathogen noxa. This may be due to their peculiar morphology; the stratified squamous epithelium of the pars esophagea is devoid of mucous-producing glands and lacks the sodium bicarbonate buffering system present in the gastric glandular mucosa, thus favouring the occurrence of damage caused by gastric acid content [2,7,13]. Indeed, it has been reported that H. suis mainly colonizes the antrum and the fundic gland zone and, to a lesser extent, the cardiac gland zone [4,13,17]. In the fundic gland region of pigs experimentally or naturally infected with H. suis, these microorganisms were found in close contact with parietal cells, which indicates that the bacterium may have an impact on hydrochloric acid-producing cells [13,17]. The secretion of excessive amounts of gastric acid may lead to increased contact of the non-glandular part of the stomach with hydrochloric acid. Consequently, this chronic insult of the non-glandular part may result in the development of ulcers. The porcine stomach physiologically maintains the proximal and the distal compartments with distinct pH and enzymatic conditions [13].
Our study did not detect H. pylori in the samples analyzed by PCR. Although Krakowka et al. [20] reported that H. pylori is able to colonize the gastric mucosa of experimentally infected pigs, there are no indications that swine are a natural reservoir of this pathogen [8,13]. To assess an in vivo detection method for H. suis, PCR analyses with specific primers were carried out on saliva and feces samples. In our study 60% and 64% of feces and saliva samples were positive in the Helicobacter spp.. PCR respectively, while they were negative for H. suis and H. pylori DNA. Sequence analysis from 8 positive samples of feces and from 8 positive samples of saliva showed 99% homology with Helicobacter spp.. Our data are in accordance with the results of other authors. Indeed, Hänninen et al. [15,16] isolated H. bilis and H. trogontum from feces samples while H. suis has never been detected.
In conclusion our results show that although PCR is effective for the detection of H. suis in gastric samples, it might be less sensitive in saliva and feces samples. There are indications that Helicobacter spp. different from H. suis, may be present in feces and saliva.
Figures and Tables
Fig. 1
Light microscopy of pars esophagea of swine stomach. Loss of epithelium in mucosa and necrosis of submucosa. U: ulcer; E: epithelium; GT: granulation tissue. H&E stain, ×100.
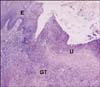
Fig. 2
PCR amplification products. (A) PCR of Helicobacter spp. Lane M: 100 bp DNA ladder; Lanes 1-4: PCR products of gastric samples, Lane 5: negative control. (B) PCR of Helicobacter suis. Lane M: 100 bp DNA ladder, Lanes 1-7: PCR products of gastric samples, Lane 8: negative control. (C) Enzymatic digestion with MboI of the Helicobacter spp. PCR product. Lane M: 100 bp DNA ladder, Lanes 1-2: Helicobacter spp. PCR products of H. pylori ATCC 43504 with (Lane 1) and without (Lane 2) MboI digestion; Lanes 3-4: Helicobacter spp. PCR products of a porcine gastric sample with (Lane 3) and without (Lane 4) MboI digestion.
Acknowledgements
Dr. Bietta was supported by European Union, European Social Fund, Ministero del Lavoro e delle Politiche Sociali and Umbria Region.
References
1. Ayles HL, Friendship RM, Ball RO. Effect of dietary particle size on gastric ulcers, assessed by endoscopic examination, and relationship between ulcer severity and growth performance of individually fed pigs. Swine Health Prod. 1996. 4:211–216.
2. Baele M, Decostere A, Vandamme P, Ceelen L, Hellemans A, Mast J, Chiers K, Ducatelle R, Haesebrouck F. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol. 2008. 58:1350–1358.

3. Baldwin DN, Shepherd B, Kraemer P, Hall MK, Sycuro LK, Pinto-Santini DM, Salama NR. Identification of Helicobacter pylori genes that contribute to stomach colonization. Infect Immun. 2007. 75:1005–1016.

4. Barbosa AJA, Silva JCP, Nogueira AMMF, Paulino E, Miranda CR. Higher incidence of Gastrospirillum sp. in swine with gastric ulcer of the pars oesophagea. Vet Pathol. 1995. 32:134–139.

5. Choi YK, Han JH, Joo HS. Identification of novel Helicobacter species in pig stomachs by PCR and partial sequencing. J Clin Microbiol. 2001. 39:3311–3315.

6. De Groote D, van Doorn LJ, Ducatelle R, Verschuuren A, Haesebrouck F, Quint WG, Jalava K, Vandamme P. 'Candidatus Helicobacter suis', a gastric helicobacter from pigs, and its phylogenetic relatedness to other gastrospirilla. Int J Syst Bacteriol. 1999. 49:1769–1777.

7. Dore MP, Bilotta M, Vaira D, Manca A, Massarelli G, Leandro G, Atzei A, Pisanu G, Graham DY, Realdi G. High prevalence of Helicobacter pylori infection in shepherds. Dig Dis Sci. 1999. 44:1161–1164.
9. Fox JC. Non-human reservoirs of Helicobacter pylori. Aliment Pharmacol Ther. 1995. 9:Suppl. 93–103.
10. Friendship RM. Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ, editors. Gastric ulcer. Disease of Swine. 2006. 9 th ed. Ames: Blackwell;891–900.
11. Grasso GM, Ripabelli G, Sammarco ML, Ruberto A, Iannitto G. Prevalence of Helicobacter-like organisms in porcine gastric mucosa: a study of swine slaughtered in Italy. Comp Immunol Microbiol Infect Dis. 1996. 19:213–217.

12. Guise HJ, Carlyle WWH, Penny RHC, Abbott TA, Riches HL, Hunter EJ. Gastric ulcers in finishing pigs: their prevalence and failure to influence growth rate. Vet Rec. 1997. 141:563–566.

13. Haesebrouck F, Pasmans F, Flahou B, Chiers K, Baele M, Meyns T, Decostere A, Ducatelle R. Gastric Helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin Microbiol Rev. 2009. 22:202–223.

14. Harding JCS, Clark EG, Strokappe JH, Willson PI, Ellis JA. Postweaning multisystemic wasting syndrome: Epidemiology and clinical presentation. Swine Health Prod. 1998. 6:249–254.
15. Hänninen ML, Kärenlampi RI, Koort JM, Mikkonen T, Björkroth KJ. Extension of the species Helicobacter bilis to include the reference strains of Helicobacter sp. flexispira taxa 2, 3 and 8 and Finnish canine and feline flexispira strains. Int J Syst Evol Microbiol. 2005. 55:891–898.

16. Hänninen ML, Utriainen M, Happonen I, Dewhirst FE. Helicobacter sp. flexispira 16S rDNA taxa 1, 4 and 5 and Finnish porcine Helicobacter isolates are members of the species Helicobacter trogontum (taxon 6). Int J Syst Evol Microbiol. 2003. 53:425–433.

17. Hellemans A, Chiers K, Decostere A, De Bock M, Haesebrouck F, Ducatelle R. Experimental Infection of Pigs with 'Candidatus Helicobacter suis'. Vet Res Commun. 2007. 31:385–395.

18. Hellemans A, Chiers K, De Bock M, Decostere A, Haesebrouck F, Ducatelle R, Maes D. Prevalence of 'Candidatus Helicobacter suis' in pigs of different ages. Vet Rec. 2007. 161:189–192.

19. Henry SC. Gastric ulcers. Feed management is top priority for prevention. Large Animal Vet. 1996. 8–11.
20. Krakowka S, Eaton KA, Rings DM. Occurrence of gastric ulcers in gnotobiotic piglets colonized by Helicobacter pylori. Infect Immun. 1995. 63:2352–2355.

21. Krakowka S, Ellis J. Reproduction of severe gastroesophageal ulcers (GEU) in gnotobiotic swine infected with porcine Helicobacter pylori-like bacteria. Vet Pathol. 2006. 43:956–962.

22. Lage AP, Godfroid E, Fauconnier A, Burette A, Butzler JP, Bollen A, Glupczynski Y. Diagnosis of Helicobacter pylori infection by PCR: comparison with other invasive techniques and detection of cagA gene in gastric biopsy specimens. J Clin Microbiol. 1995. 33:2752–2756.

23. Melnichouk SI, Friendship RM, Dewey CE, Bildfell RJ, Smart NL. Helicobacter-like organisms in the stomach of pigs with and without gastric ulceration. Swine Health Prod. 1999. 7:201–205.
24. Mendes EN, Queiroz DMM, Rocha GA, Nogueira AMMF, Carvalho ACT, Lage AP, Barbosa AJA. Histopathological study of porcine gastric mucosa with and without a spiral bacterium ("Gastrospirillum suis"). J Med Microbiol. 1991. 35:345–348.

25. Moyaert H, Pasmans F, Ducatelle R, Haesebrouck F, Baele M. Evaluation of 16S rRNA gene-based PCR assays for genus-level identification of Helicobacter species. J Clin Microbiol. 2008. 46:1867–1869.

26. Park JH, Lee BJ, Lee YS, Park JH. Association of tightly spiraled bacterial infection and gastritis in pigs. J Vet Med Sci. 2000. 62:725–729.

27. Park JH, Seok SH, Cho SA, Baek MW, Lee HY, Kim DJ, Park JH. The high prevalence of Helicobacter sp. in porcine pyloric mucosa and its histopathological and molecular characteristics. Vet Microbiol. 2004. 104:219–225.

28. Queiroz DMM, Rocha GA, Mendes EN, De Moura SB, De Oliveira AMR, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996. 111:19–27.

29. Riley LK, Franklin CL, Hook RR Jr, Besch-Williford C. Identification of murine helicobacters by PCR and restriction enzyme analyses. J Clin Microbiol. 1996. 34:942–946.

30. Robertson ID, Accioly JM, Moore KM, Driesen SJ, Pethick DW, Hampson DJ. Risk factors for gastric ulcers in Australian pigs at slaughter. Prev Vet Med. 2002. 53:293–303.

31. Roosendaal R, Vos JH, Roumen T, van Vugt R, Cattoli G, Bart A, Klaasen HL, Kuipers EJ, Vandenbroucke-Grauls CM, Kusters JG. Slaughter pigs are commonly infected by closely related but distinct gastric ulcerative lesion-inducing gastrospirilla. J Clin Microbiol. 2000. 38:2661–2664.

32. Sgalés J, Sitjar M, Domingo M, Dee S, Del Pozo M, Noval R, Sacristan C, De las Heras A, Ferro A, Latimer K. First report of post-weaning multisystemic wasting syndrome in pigs in Spain. Vet Rec. 1997. 141:600–601.
33. Szeredi L, Palkovics G, Solymosi N, Tekes L, Méhesfalvi J. Study on the role of gastric Helicobacter infection in gross pathological and histological lesions of the stomach in finishing pigs. Acta Vet Hung. 2005. 53:371–383.

34. Thomson MA, Storey P, Greer R, Cleghorn GJ. Canine-human transmission of Gastrospirillum hominis. Lancet. 1994. 343:1605–1607.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download