Abstract
Autoantibodies against thyroxin (T4AA) and triiodothyronine (T3AA) are present in dogs with autoimmune thyroiditis and have been reported to interfere with immunoassays. The objectives of this study were to determine the frequency of autoantibodies and to determine whether interference occurs by T4AA, using a non-immunological method (high performance liquid chromatography, HPLC) for thyroxin (T4) measurement. Based on clinical symptoms, T4 and thyroid stimulating hormone (TSH) concentration, 1,339 dogs were divided into six groups: Group 1: hypothyroid (n = 149); Group 2: subclinical thyroiditis (n = 110); Group 3: suspicious for non thyroidal illness (n = 691); Group 4: biochemical euthyroid (n = 138); Group 5: hypothyroid dogs under substitution therapy (n = 141); Group 6: healthy dogs (n = 110). The incidence of T4AA and T3AA, determined using radiometric assay, was low (0.5% and 3.8%) and higher in hypothyroid dogs compared to dogs suspicious for hypothyroidism (Group 2-4) (p<0.05). T4AA was not detected in dogs with normal T4 and elevated TSH. T4 concentrations of T4AA positive samples determined using HPLC were comparable to results obtained by chemiluminescence immunoassay. These findings indicate that the probability of interference of T4AA leading to falsely elevated T4 concentration in the T4 assay seems to be low.
Autoimmune thyroiditis (AIT) is a frequent cause of primary hypothyroidism, which is one of the most common endocrinopathies in dogs. Autoantibodies against thyroglobulin (TgAA), thyroid hormones (THAA) and thyroid peroxidase have been detected in the circulation of dogs with hypothyroidism caused by AIT [11,19]. However thyroxin (T4) and triiodothyronine (T3) are small molecules with a molecular weight of <1,000 Dalton and therefore do not stimulate an immune response by themselves unless they are covalently linked to an immunogen like thyroglobulin. Thus, TgAA cross react with T4 and/or T3 as these molecules are covalently bound to thyroglobulin [17,21]. It has been reported that autoantibodies against triiodothyronine (T3AA) and thyroxin (T4AA) in serum samples of dogs with AIT may interfere with the immunological assay methods used to measure thyroid hormone concentration, leading to falsely elevated or reduced concentrations, depending on the assay method [14,18]. The measurement of TgAA is considered useful as they are associated with AIT and THAA is recommended in cases of doubtful T4 results [8]. In dogs, falsely elevated T4 concentrations in the presence of T4AA have been reported for a commercially available T4 radioimmunoassay (RIA) [14]. Thus, T4 could falsely lie in the normal reference range in a hypothyroid dog with T4AA, and a T4 concentration within the normal reference range is normally considered to rule out hypothyroidism [13].
Although the reported prevalence of T4AA in dogs with clinical signs of hypothyroidism is low (between 0.6% and 4.0%), the interference of autoantibodies with hormone measure is a subject of much debate, especially for cases in which the hormone measurements do not fit the clinical sign [1,5,14,16,20,22]. Nachreiner et al. [14] described that in 17 of 1,000 hypothyroid dogs, T4AA caused a falsely elevated T4 concentration as measured by RIA, but nothing was known about the actual hormone concentration in these dogs. Moreover, THAA also appears in dogs with subclinical stages of AIT in which T4 concentrations often remain within the reference range [11]. Therefore, the first objective of this study was to determine the prevalence in Germany of THAA in dogs with clinical signs of hypothyroidism, normal T4 and elevated thyroid stimulating hormone (TSH) concentrations. Autoantibodies were also determined in hypothyroid dogs substituted with levothyroxine (predominantly Forthyron, Albrecht, Germany) and clinically healthy dogs. Second, a non-immunologic method (high performance liquid chromatography, HPLC) for the measurement of T4 in T4AA positive serum samples was established to determine the possible interference of T4AA in an immunological based T4 assay.
A total of 1,339 serum samples from dogs of various breeds, ages and both sexes was received from practitioners throughout Germany and allocated into six groups. Samples were kept frozen at -20℃ until assay for T4AA, T3AA, T4 and TSH. Laboratory measurements were done in the endocrinology laboratory of the University of Veterinary Medicine in Hannover, Germany. Dogs (n = 1,088) with at least three clinical symptoms of hypothyroidism (lethargy, weight gain, poor hair coat, pyoderma, weakness, bradycardia, diarrhea, constipation, anaemia, hyperlipidemia) were allocated into four groups on the basis of serum T4 and TSH concentration. The laboratory reference range for T4 was 1.7~4.5 µg/dL (21.7~57.9 nmol/L), while concentrations between 1.1~1.6 µg/dL (14.2~20.6 nmol/L) were designated borderline. TSH concentrations <0.5 ng/mL were considered physiological. The dogs of Group 1 (n = 149, age = 7.5 ± 5.6) were defined as hypothyroid (T4<1.1 µg/dL, TSH>0.5 ng/dL); those of Group 2 (n = 110, age = 7.2 ± 3.4) were classified as suspicious for hypothyroidism, subclinical thyroiditis or T4AA interference (T4>1.1 µg/dL, TSH>0.5 ng/dL); those of Group 3 (n = 691, age = 6.9 ± 3.2) were classified as non-thyroidal illness or hypothyroidism without elevated TSH (T4<1.7 µg/dL, TSH<0.5 ng/dL). Dogs with normal T4 and TSH concentrations were classified as biochemical euthyroid (Group 4; n = 138, age = 5.8 ± 2.9). Hypothyroid dogs (n = 141, age = 8.0 ± 2.9) under substitution therapy with levothyroxine were assigned to Group 5. The duration of the substitution therapy ranged between 3 months and 4.5 years. In T3AA positive dogs a follow up measurement over 9 months was possible. Clinically healthy dogs of various breeds (Beagle 26%; Terriers 10%, Boxer 7%, Dachshund 7%, German Shepherd 8%, English Bulldog 5%, Large Swiss mountain dog 4%, Labrador Retriever 3%, Border collie 2% and other 43%; n = 110, age = 5.1 ± 2.2) from breeding programs were allocated into Group 6. T4AA negative serum samples for HPLC measurement (n = 5) from clinically healthy dogs were made available from another project. T4AA positive serum samples for HPLC analysis (n = 6, sufficient volumes for repeated analyses) were donated by Prof. Dr. R. Nachreiner, Michigan State University, USA.
Autoantibodies were determined using a radiometric assay based on the binding of radiolabelled T4 or T3 to serum samples and separating of the bound and free fractions by charcoal treatment [14]. Serum samples (100 µL) were incubated with 0.02 µCi of 125I-T4 ([125I] - Thyroxin, specific activity 5,700 µCi/µg; Perkin Elmer, USA) or with 0.02 µCi of 125I-T3 ([125I]-Triiodothyronine, specific activity 3,300 µCi/µg; Perkin Elmer, USA) in 500 µL of phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) containing 5 mM of 8 - anilino - 1 naphtalene sulfonic acid for 1 h at 37℃ and for 1 h at 4℃. Charcoal suspension (500 µL; equates to 15 mg activated charcoal per tube; Sigma Aldrich, USA) was added. The mixture was incubated for 10 min at 4℃ and then centrifuged for 10 min at 2,000 × g and 4℃. The supernatant was decanted and the radioactivity in the remaining pellet was counted in a gamma counter (LKB-Wallac Clini-Gamma 1272; LKB-Wallac, Finland). The percentage of binding was calculated using the formula [(T-P)/T] × 100, with T = total activity incubated and P = activity in the pellet. Based on a study of Nachreiner et al. [14] a threshold of 10% - binding for T3AA and 20% - binding for T4AA was chosen to discriminate between positive and negative test results. For validation, pooled serum from T4AA and T3AA positive dogs, respectively, were used as positive controls. Serum from clinically healthy dogs with thyroid hormone concentrations within the reference range was pooled and used as autoantibody-negative control. The accuracy of the radiometric autoantibody-assay was tested by repeated analysis of positive and negative controls. The intra-assay coefficient of variation (CV%) was 0.8% for T3AA-positive controls and 3.3% for T4AA-positive controls. The inter-assay CV% was 7.1% for the T3AA-positive sample and 9.6% for the T4AA-positive sample. The negative control serum was at all times negative for T3AA (<10% - binding) and for T4AA (<20% - binding).
T4 and TSH were determined using a commercially available chemiluminescence immunoassay (CIA, Immulite canine T4, LKCT5 and LKKT5; Siemens Medical Solutions, USA). The measurement was performed according to the manufacturer's instructions. The intra-assay CV% for the T4 assay varied between 3.9% and 10.8%, while the inter-assay CV% was between 5.2% and 13.8%. For TSH measurement, the intra-assay CV% ranged between 3.8 and 5.0% and the inter-assay CV% ranged between 6.3 and 8.2%. The analytical sensitivity for T4 was 0.12 µg/dL (1.5 nmol/L) and for TSH 0.01 ng/mL.
A HPLC method has been developed for the determination of T4 in canine serum that includes hormone extraction after protein precipitation, purification of the extract by solid phase extraction reversed phase HPLC and quantification with a UV-monitor [10]. Protein was precipitated by adding 50% trifluoro acetic acid (0.5 mL, 4℃) to serum samples (0.5 mL) and centrifuging the mixture at 4℃ and 2,000 × g for 20 min. The supernatant was then extracted with 1 mL of ethyl acetate. The organic phase was concentrated to 20% of its original volume, adjusted to pH 2 with 0.02 M HCl and loaded onto a SPE cartridge containing 3 mL Strata TM-X-C (Phenomenex, USA). The T4 containing fraction was eluted with dichloromethane / 2 - propanol / methanol / 25% ammonia (30 : 30 : 30 : 5). After evaporation of the solvent the sample was redissolved in 100 µL of 90% methanol, 20 µL of which were injected into the HPLC system (UV / Vis Detector SPD - 20A and a Phenomenex Jupiter 4 µ Proteo 90 Å column 150 × 2 mm; Shimadzu, Japan). The extract was eluted at a flow rate of 0.22 mL/min using a linear solvent gradient starting with water/acetonitril 88 : 12 containing 0.025% TFA proceeding to water/acetonitril 20 : 80 containing 0.02% TFA after 50 min. For each run, 20 µL of reconstituted sample was injected. With UV absorbance detection at 228 nm and 215 nm simultaneously, T4 peaks of the same height were observed at both wavelengths after 42 min retention time. Data were evaluated using the Shimadzu LC solution software (Shimadzu, Japan). The system was calibrated using freshly prepared thyroxin standards at concentrations of 0.8, 1.0 and 1.3 µg/dL. The T4 concentration was determined in 11 autoantibody negative serum samples. The intra-assay CV% was 6.0%. The analytical sensitivity in serum samples after protein precipitation and extraction of the HPLC method was 0.5 µg/dL. The recovery was 92% if 1.0 µg/dL thyroxin was added to respective serum samples. The correlation between T4 results of HPLC and CIA in autoantibody negative serum samples was high (r2 = 0.91).
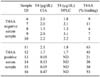
Of the 1,088 serum samples of dogs with clinical symptoms suspicious of hypothyroidism, 13.7% were allocated on the basis of their T4 and TSH concentrations to Group 1, 10.1% to Group 2, 63.5% to Group 3 and 12.7% to Group 4. The distribution of T3AA and T4AA positive dogs in the six groups is summarized in Table 1. All of three T3AA positive dogs in Group 2 had T4 concentrations between 1.1~1.7 µg/dL and elevated TSH but none of the dogs with T4 within the reference range (>1.7 µg/dL) had autoantibodies. THAA were found in this study in 20 male (3.8% of all males) and 21 female (3.2% of all females) dogs, hence no significant difference between male and female dogs was found in the frequency of autoantibodies (p = 0.949). Four of the 20 male dogs were castrated and four of the 21 female dogs were spayed; this difference was not significant. T4AA were only detected in four dogs of Group 1 and additionally in one dog of Group 3 (Table 2). Four of these five dogs had low T4 concentrations as measured by CIA. In one dog (Cairn terrier) the results for autoantibodies (T4AA and T3AA) and T4 concentrations were borderline.
The occurrence of T3AA and T4AA was comparable between dogs already substituted with thyroxin and dogs in which hypothyroidism was suspected (Group 1~4). In one dog of Group 5, the follow-up measurements of T3AA over several months revealed that the T3AA concentration gradually decreased to 37% binding after 1 month, to 26% after 5 months and finally to 19% binding 9 months after beginning the substitution therapy. Neither T3AA nor T4AA were detected in any clinically healthy dog (Group 6).
The T4 concentrations measured using both CIA and HPLC were comparable, irrespective of the presence or absence of T4AA (Table 3). In serum samples with low T4 concentrations obtained by CIA, no peak was detected in the HPLC analysis, which was equivalent to a concentration below 0.5 µg/dL.
The measurement of T4 and TSH is widely used in diagnosing primary hypothyroidism in dogs. However, these serum concentrations can be altered by non-thyroidal illness or as a side effect of certain drugs, leading to confusing results and a more difficult diagnosis, even if clinical signs suggestive of hypothyroidism are present [8]. THAA have been suspected of interfering with immunological hormone assays, leading to falsely elevated T4 concentration and thereby inappropriately ruling out hypothyroidism [5,14]. TgAA have been detected in approximately 50% of dogs with a AIT [1,11], and have been reported to be associated with autoantibodies directed against T4 and T3 [9].
In the present study, T3AA and T4AA were detected in 3.8% and 0.5%, respectively, of samples from dogs with clinical signs suggestive of hypothyroidism. These figures are comparable to those of Nachreiner et al. [14], who reported that 4.6% and 0.6% of dogs with suspected hypothyroidism were positive for T3AA and T4AA, respectively. Accordingly, T4AA seem to be rarer compared to T3AA in our study, which is in general agreement with other published data [9,14,16,20,22]. In contrast to the findings of Nachreiner et al. [14] who detected significantly more THAA in females than males, there was no evidence of gender influencing the prevalence of THAA in the present study. This may be due to different selection criteria and the smaller sample size analyzed in the present study. Significantly more dogs from Group 1 (clinical signs of hypothyroidism, low T4, elevated TSH) were positive for THAA compared to clinically suspicious dogs with questionable hormone concentrations (Group 2 and 3) or hormone concentrations within the laboratory reference range (Group 4).
However, the incidence of THAA in dogs with different onset of AIT and different severity of clinical signs were difficult to compare because in the final stage of thyroid destruction production of autoantibodies ceases. The presence of THAA in Group 2~4 could be interpreted as early signs of AIT. This finding is in agreement with Graham et al. [11] who suggested that TgAA can be measured in dogs developing hypothyroidism caused by AIT but still have enough functional thyroid tissue to provide normal or only slightly decreased T4 concentrations without increased TSH. These dogs may already have early clinical signs consistent with hypothyroidism like lethargy, increased body weight and poor hair coat even with only slightly lowered or normal T4 concentrations.
In humans with AIT, a slightly increased risk for developing T4AA under substitution therapy with T4 has been suggested [3], but in the present study none of the already substituted dogs were positive for T4AA. Similar to other data in the literature about TgAA [16], the percentage of T3AA binding of one dog in our study was reduced during a 9 month follow-up.
In contrast to previous findings about the occurrence of TgAA in clinically healthy dogs [7], in the present study no clinically healthy dog was positive for THAA.
THAA determinations are recommended when clinical signs do not match with laboratory results [13]. However, the possible interference of THAA with different immunological and non-immunological hormone measurement methods has not been properly elucidated in dogs. Four of five T4AA positive dogs of the present study had low T4 concentrations as measured by CIA. No dog with clinical signs due to hypothyroidism and normal T4 but elevated TSH concentrations was positive for T4AA (Group 2). This result may undermine the hypothesis that T4AA interferences can potentially lead to falsely elevated T4 concentration and confuse the diagnosis of hypothyroidism.
In humans, T4 determination using HPLC is an established reference method for validating assays and the technique has been widely applied to the measurement of iodothyronines [4,10]. The T4 concentrations of T4AA positive samples as analysed by HPLC revealed good agreement with the immunological T4 assay results irrespective of T4AA titer. This result may indicate that the CIA is less sensitive to T4AA interference. This is the first time that T4AA positive serum samples have been assayed for T4 using HPLC and an immunoassay. However, the magnitude of the results was not enough to make a universally valid prediction about T4AA interferences in chemiluminescence assays. However, interference seems unlikely. No dog suggestive for subclinical thyroiditis (normal T4, elevated TSH) was positive for T4AA. Moreover the T4 concentration in T4AA positive samples obtained by HPLC and immunoassay showed a good correlation. In a previous study a direct linear correlation was observed between T3AA and apparent serum T3 concentrations as measured by RIA, indicating a possible interference of the autoantibodies with the RIA [9]. If the radioactive tracer (T3 labelled with 125I) binds to the autoantibodies in serum as well as to the assay antibodies a falsely increased value may occur, depending on the affinity of the assay and autoantibodies respectively. In a solid phase RIA, paradoxically low T4 was measured [12]. Theoretically, the CIA used in this study should also have shown falsely elevated T4 concentrations; however, not every assay for T4 will be influenced in the same way because antibodies with different affinities and binding characteristics as well as different separation methods are employed by manufacturers. Presumably, immunoassay with monoclonal antibodies is less sensitive to interference by autoantibodies than the RIA system with polyclonal antibodies [14]. However, it should be noted that Nachreiner et al. [14] found that only 17 of 1,000 T4AA positive samples from hypothyroid dogs were likely to be falsely elevated. Ferguson et al. [8] concluded that antibodies only interfere with the immunoassay if high autoantibody titers are reached. In the present study, one sample had a high T4AA titer (>50%) but T4 concentrations obtained by CIA and HPLC were comparable. This reveals that a respective bias is most unlikely. Furthermore, varying affinities of the detected autoantibodies have been described in humans, which could explain different influences in assay systems [2,6,12,15].
Further studies with T4AA positive serum samples are needed to confirm these data, particularly in view of the reported differences in autoantibody affinities.
In conclusion this study was the first survey in Germany on the prevalence of thyroid hormone autoantibodies in treated (levothyroxine) and non-treated hypothyroid dogs and dogs suspected to be hypothyroid, and the results were comparable to published data. When applying HPLC analysis to T4AA positive serum samples, no falsely elevated T4 values as measured by CIA could be verified. Furthermore, no T4AA was detected in the group of dogs with normal T4 and elevated TSH, indicating that the expected interference of T4AA did not feature in the present study. The data from the present study suggest that, when using a comparable chemiluminescence immunoassay, analysis of T4AA does not provide more information than TGAA for the diagnosis of hypothyroidism in dogs.
Figures and Tables
Acknowledgments
The authors thank Prof. Dr. R. Nachreiner, Michigan State University, East Lansing, MI, USA for providing autoantibody positive serum samples.
References
1. Beale KM, Halliwell RE, Chen CL. Prevalence of antithyroglobulin antibodies detected by enzyme-linked immunosorbent assay of canine serum. J Am Vet Med Assoc. 1990. 196:745–748.
2. Beck-Peccoz P, Romelli PB, Faglia G. Circulating antitriiodothyronine autoantibodies in two euthyroid patients: apparent lack of interference in total T3 radioimmunoassay based on second antibody or solid phase separation techniques. J Endocrinol Invest. 1983. 6:333–340.

3. Biuković M, Musafija A, Skrobića M, Golubovic N, Mikać Z, Rajkovaca Z, Zubović I. Autoantibodies to triiodothyronine and thyroxine in primary hypothyreosis. Med Pregl. 1993. 46:Suppl 1. 80–81.
4. Burman KD, Bongiovanni R, Garis RK, Wartofsky L, Boehm TM. Measurement of serum T4 concentration by high performance liquid chromatography. J Clin Endocrinol Metab. 1981. 53:909–912.

5. Chastain CB, Young DW, Kemppainen RJ. Anti-triiodothyronine antibodies associated with hypothyroidism and lymphocytic thyroiditis in a dog. J Am Vet Med Assoc. 1989. 194:531–534.
6. De Baets M, Sels J, van Breda Vriesman P, Elewaut A, Vermeulen A, Willems P, Coenegracht J. Monoclonal triiodothyronine (T3)-binding immunoglobulins in a euthyroid woman. Clin Chim Acta. 1982. 118:293–301.

7. Deeg C, Kaspers A, Hartmann K 2nd, Kraft W, Kaspers B. Canine hypothyroidism: detection of anti-thyroglobulin autoantibodies. Tierarztl Prax. 1997. 25:170–173.
8. Ferguson DC. Testing for hypothyroidism in dogs. Vet Clin North Am Small Anim Pract. 2007. 37:647–669.

9. Gaschen F, Thompson J, Beale K, Keisling K. Recognition of triiodothyronine-containing epitopes in canine thyroglobulin by circulating thyroglobulin autoantibodies. Am J Vet Res. 1993. 54:244–247.
10. Gika H, Lämmerhofer M, Papadoyannis I, Lindner W. Direct separation and quantitative analysis of thyroxine and triiodothyronine enantiomers in pharmaceuticals by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2004. 800:193–201.

11. Graham PA, Nachreiner RF, Refsal KR, Provencher-Bolliger AL. Lymphocytic thyroiditis. Vet Clin North Am Small Anim Pract. 2001. 31:915–933. vi-vii.

12. Heyma P, Harrison LC. Autoantibodies to thyroid hormones: association with falsely low hormone levels measured by 'Amerlex' assay. Clin Chim Acta. 1986. 161:239–242.

13. Kemppainen RJ, Young DW, Behrend EN, Clark TP, Smiley SD. Autoantibodies to triiodothyronine and thyroxine in a golden retriever. J Am Anim Hosp Assoc. 1996. 32:195–198.

14. Nachreiner RF, Refsal KR, Graham PA, Bowman MM. Prevalence of serum thyroid hormone autoantibodies in dogs with clinical signs of hypothyroidism. J Am Vet Med Assoc. 2002. 220:466–471.

15. Neeley WE, Alexander NM. Polyclonal 3,5,3'-triiodothyronine (T3) antibodies in a euthyroid woman and their effect on radioimmunoassays for T3. J Clin Endocrinol Metab. 1983. 57:851–854.

16. Patzl M, Möstl E. Determination of autoantibodies to thyroglobulin, thyroxine and triiodothyronine in canine serum. J Vet Med A Physiol Pathol Clin Med. 2003. 50:72–78.

17. Pearce CJ, Byfield PG, Edmonds CJ, Lalloz MR, Himsworth RL. Autoantibodies to thyroglobulin cross reacting with iodothyronines. Clin Endocrinol (Oxf). 1981. 15:1–10.

18. Savastano S, Tommaselli AP, Valentino R, Dorato M, Scarpitta MT, Persechino A, Ciaramella P, Lombardi G. Usefulness of a chromatographic method to detect circulating antithyroid hormone autoantibodies in canine serum. J Endocrinol Invest. 1996. 19:758–762.

19. Skopek E, Patzl M, Nachreiner RF. Detection of autoantibodies against thyroid peroxidase in serum samples of hypothyroid dogs. Am J Vet Res. 2006. 67:809–814.

20. Thacker EL, Refsal KR, Bull RW. Prevalence of autoantibodies to thyroglobulin, thyroxine, or triiodothyronine and relationship of autoantibodies and serum concentrations of iodothyronines in dogs. Am J Vet Res. 1992. 53:449–453.
21. Young DW, Kemppainen RJ, Sartin JL. Characterization of canine triiodothyronine (T3) autoantibodies and their effect on total T3 in canine serum. Proc Soc Exp Biol Med. 1988. 188:219–228.

22. Young DW, Sartin JL, Kemppainen RJ. Abnormal canine triiodothyronine-binding factor characterized as a possible triiodothyronine autoantibody. Am J Vet Res. 1985. 46:1346–1350.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download