Abstract
Distribution and characterization of interlukin-10 (IL-10)-secreting cells in lymphoid tissues of pigs naturally infected with porcine circovirus type 2 (PCV2) were evaluated in accordance with PCV2 antigen detection. After screening a total of 56 pigs showing the symptoms of postweaning multisystemic wasting syndrome (PMWS), 15 pigs were PCV2 positive and 5 pigs, which showed stronger positive signals over multiples tissues were further investigated. This study showed that in PCV2-infected lymphoid tissues, particularly mandibular lymph node, spleen and tonsil, IL-10 expression was mainly localized in T-cell rich areas but rarely in B cell rich areas. IL-10 was highly expressed in bystander cells but rarely in PCV2-infected cells. Elevated IL-10 expression was predominantly associated with T cells, but rarely with B cells or with macrophages. The results of this study provide evidence for the role of IL-10 in chronic PCV2 infection and its relation to PCV2 antigen in affected tissues. Constantly elevated levels of IL-10 lead to immunosuppression in persistent and chronic viral infections. The increased IL-10 expression observed in PCV2 infection in this study suggests that IL-10-mediated immunosuppression may play an important role in the pathogenesis and maintenance of naturally occurring PCV2 infection.
Porcine circovirus type 2 (PCV2) is a non-enveloped virus with a 1.76 Kb single-stranded DNA genome belonging to the genus Circovirus, family Circoviridae, and is found in tissues of almost all cases of postweaning multisystemic wasting syndrome (PMWS). PCV2 is considered ubiquitous in both domestic and wild swine [1,23]. Diagnosis of PMWS is based on three criteria: clinical, histopathological, and in situ detection of PCV2 by immunohistochemistry (IHC) in the lymphoid tissues of affected animals. Microscopically, PMWS is characterized by lymphoid depletion, cytoplasmic viral inclusions, histocytic infiltration, and presence of syncytial cells in lymphoid tissues [4,16,18,20]. Clinical signs of the disease are generally non-specific; however, in some cases severe and often fatal conditions are observed. Clinical signs often include chronic wasting and respiratory disease. Moreover, PCV2 infection may be substantially immunosuppressive, allowing secondary opportunistic bacterial and viral infections to develop [5,7,13,19].
Chronic or persistent viral infections are reported to induce high interleukin-10 (IL-10) production which may prevent viral clearance by causing profound immunosuppression [3]. IL-10 was originally identified as a T helper type 2 (Th2) cytokine and it is now well established as having potent anti-inflammatory activity during infection. IL-10 is produced by Th2 cells, subsets of regulatory T cells designated Tr1, Th1, Th17 and CD8+ cells [14,15]. Other important sources of IL-10 include monocytes, stimulated macrophages, some subsets of dendritic cells, as well as human B cells [15]. Previous studies have reported that PCV2-infected pigs suffer severe lymphopenia and altered cytokine production in peripheral blood mononuclear cells and lymphoid tissues. These studies indicated that increased levels of IL-10 mRNA expression occurred in the thymus and decreased levels of IFN-γ and IL-4 mRNA were seen in other lymphoid tissues after exposure to PCV2 [7-9,13,21]. Elevated serum levels of IL-10 were detected in PCV2-infected piglets that subsequently developed PMWS [22]. It is not known which cell populations are the principal producers of IL-10 in infected swine. There have been no morphological studies to determine the location of anti-inflammatory cytokines especially among T or B cells in naturally PCV2-infected pigs.
In the preliminary experiments of IL-10 and PCV2 double labeling immunohistochemistry, there was very minimal co-localization detected between IL-10 and PCV2 antigen expressions in cells detected in T-cell rich areas. Moreover, originally IL-10 antigen staining was observed in cells located spatially in juxtaposition to PCV2 positive cells. These findings suggest that PCV2 induces IL-10 secretion predominantly in bystander cells such as T-cells and non-infected macrophages in infected lymphoid tissues. In the present study, we evaluated the distribution of IL-10 antigen expressed in lymphoid tissues, including mandibular lymph nodes, spleens, and tonsils. In addition, the subpopulation of IL-10 producing cells among T and B cells and their localization was examined in relation to PCV2-infected cells. This is the first report regarding the tissue distribution and characterization of the IL-10-producing cells in lymphoid tissues of pigs naturally infected with PCV2.
The materials for diagnostic investigation were submitted from 3 different time points. A total of 56 pigs from five different farms (22 naturally PMWS suspected animals originating from 3 different herds, 16 from the fourth herd and 18 from the fifth herd) underwent routine diagnosis at an age of between 2 to 4 weeks. Over the past year, the animals from these five farms have had a history of relatively poor hygiene, poor sanitation, and most importantly a history of PMWS or respiratory distress. We selected 15 individual pigs based on the presence of all of the following criteria: 1) clinical conditions of wasting, dyspnea, enlarged lymph nodes, and icterus; 2) histopathology of extensive loss of the normal follicular architecture with depletion of lymphocytes from T/B cell regions and infiltrate of histiocytic cells intermixed with granulomatous inflammatory cells (multinucleated giant cells) in lymphoid tissues [16,17]; 3) IHC for PCV2. All pigs were subjected to necropsy in the Department of Veterinary Pathology, Konkuk University, Korea. Various tissue sections from tonsil, spleen, liver, kidney, and mandibular, mesenteric, and inguinal lymph nodes were prepared, and the samples were fixed in neutral buffered formalin subsequently. Representative hematoxylin and eosin-stained slides from these tissue sections were also prepared. Among those 15 pigs, only five pigs consistently showed moderate to strong positive signals for PCV2 antigens in at least three different lymphoid tissues. Tissue sections from mandibular lymph nodes, spleen, and tonsil of these five selected pigs were consistently PCV2 antigen-positive by immunohistochemical staining. In addition, five more age-matched pigs that were proven negative for PCV2 and PRRSV by immunohistochemical staining were selected and corresponding tissue sections were prepared to serve as controls.
Routine IHC was performed using formalin-fixed, paraffin-embedded sections. Slides were placed in 65℃ oven for 20 min, deparaffinized and rehydrated through xylene and graded ethanol solutions to phosphate-buffered saline (PBS, pH 7.4). Slides for horseradish peroxidase (HRP) were quenched for 20 min in a 3% hydrogen peroxide solution in PBS to block endogenous peroxidase. After three further washes in PBS, antigens were retrieved either by a heat method in which the specimens were placed in citric acid, pH 6.0, for 20 min in a microwave oven (650 W at high power setting) or digested with 0.05% Protease XIV (Sigma, USA) in PBS for 7 min at 37℃ as per the manufacturer's instructions. After cooling, slides were washed three times in PBS and sections were incubated in a blocking solution (5% normal donkey serum for IL-10 or 5% normal goat serum for PCV2) for 30 min at room temperature. After the pretreatment conditions, the following antibody dilutions and treatment were used: anti-porcine IL-10 antibody (1 : 50, 0.05% protease XIV; R&D Systems, USA), anti-swine CD3 (1 : 100, a citric acid pretreatment; VMRD, USA), anti-human CD79a (1 : 100, a citric acid pretreatment; DakoCytomation, USA), polyclonal anti-PCV2 rabbit antibody (1 : 200, 0.05% Protease XIV; Iowa State University, USA). Sections were incubated either at 4℃ overnight (IL-10, CD3, CD79a) or at room temperature for 2 h (PCV2). In order to visualize immunolabelling, a two-step G/2 Envision system (DakoCytomation, USA) was applied after the removal of the primary antibodies. In this system, the Envision rabbit/mouse reagents conjugated to either horseradish peroxidase (HRP-system) or alkaline phosphatase (AP-system) were applied for 30 min at room temperature. The slides were subsequently washed three times in PBS and incubated with the supplied substrates until the desired color intensity developed. The reaction was stopped by washing in distilled water. Sections were counterstained with Harris's hematoxylin. In addition, a DL-IHC technique (DakoCytomation, USA) for single-tissue sections was performed to assay for co-localization of IL-10 and CD3, IL-10 and CD79a, and IL-10 and PCV2.
In order to do statistical analysis based on stained slides, 15-19 fields were randomly selected using random number tables and the number of positive cells was manually counted in each field. The statistical significance between infected and control slides was calculated by Student's t unpaired test. A p value of less than 0.05 was considered significant.
For this study, the diagnosis of a case with naturally occurring chronic PCV2 infection was based on 3 main criteria [16,17]: 1) specific clinical signs, 2) lesions in lymph nodes and 3) presence of PCV2 viral antigens. In our study, clinical signs of affected pigs, including wasting, dyspnea, and icterus, were particularly suggestive. Circovirus infection had previously been diagnosed in these herds. Clinical signs in sampled pigs were identical to those noted in pigs previously confirmed as positive based on gross and microscopic lesions and use of circovirus IHC. Other nonspecific clinical signs of disease were also noted. Gross lesions of pneumonia and enlargement of mandibular, tracheobronchial, mesenteric or inguinal lymph nodes were apparent in most pigs. Histopathological lesions of lung showed characteristic interstitial pneumonitis, and the lymphoid tissues in PCV2-infected pigs reveals extensive lymphoid depletion with loss of follicular architecture (B-cell and parafollicular T-cell dependent areas) and granulomatous inflammatory cells. This histopathological lesion was characterized by prominent infiltration of histiocytic cells and presence of syncytial cells. PCV2 antigens in the cytoplasm of macrophages and syncytial cells were observed in lymph nodes, tonsil and spleen.
Mandibular lymph nodes, spleens, and tonsils from all PCV2-positive pigs were examined for IL-10 expression by IHC. IL-10 signal was mainly localized in the cytoplasm of T cells and macrophage-like cells in the mandibular lymph node, spleen and tonsil. PCV2-infected tissues which contained a strong signal for PCV2 antigens by IHC had fewer numbers of cells that had less intense staining for IL-10. However, PCV2-infected tissues with moderate or weak signals for PCV2 antigens had abundant cells showing strong immunoreactivity for IL-10. IL-10 expression was most intense in the marginal zone of lymphoid follicles in mandibular lymph nodes (Fig. 1A) and in splenic nodule (Fig. 1B). Also, few positive cells were noted in germinal centers. In contrast, IL-10 signals in the tonsil were consistently localized in and around the crypt epithelium (Fig. 1C).
CD3 antigen was present in the cell membrane and cytoplasm of the normal or activated T cells found in the marginal zone of mandibular lymph nodes (Fig. 1D), spleen, and tonsil. CD79a expression was present on the surface of virtually all B lymphocytes in the germinal centers of mandibular lymph nodes (Fig. 1E), spleen and tonsil. Occasionally, CD79a expression was also found in lymphocytes in the marginal zone of T cell rich areas. PCV2 antigens in PMWS-affected tissues were detected in the cytoplasm of macrophage-like cells and syncytial cells in mandibular lymph nodes (Fig. 1F).
IL-10 distribution and its co-localization with T-/B-cells by DL-IHC are summarized in Table 1. The proportion of IL-10 positive cells to total cells in the mandibular lymph node and spleen of PCV2-infected pigs are noted in Table 2. IL-10 was expressed in the tissues of the control group with a low level (data not shown). However, the expression of IL-10 in PCV2-infected tissues was significantly higher than in control tissues (p<0.01). All tissues from suspected circovirus-infected pigs stained positive for presence of PCV2 antigens. The immunostaining patterns of IL-10 and CD3 were strong in the cytoplasm and outer cell membranes. Where both cytoplasmic IL-10 and CD3 were co-localized, the intensity of staining was greater in paracortical nodule of mandibular lymph node (Fig. 2A) and in the marginal zone of splenic nodules (Fig. 2B). The DL-IHC staining pattern of IL-10 and CD79a correlates to that of IL-10 and CD3. Both cytoplasmic expression of IL-10 and CD79a were evident and mainly present in the marginal zone of mandibular lymph nodes (Fig. 2C) and splenic nodules. However, the number of double-labeled cells was significantly less than that of double-labeled IL-10 and CD3. The expression of IL-10 and PCV2 antigens was also evaluated by DL-IHC. Both cytoplasmic PCV2 and IL-10 co-expression was mainly in the marginal zone of mandibular lymph node (Fig. 2D) and in paracortical nodule of spleen.
Several cytokines play a critical role in the regulation and activation of the adaptive immune response in various infections, inflammation, and even cancer development. For example, tumor necrosis factor α, IFN γ, IL-10, and interleukin-12 are known to play a role in pulmonary defense against porcine reproductive and respiratory syndrome virus [6,11]. Among these cytokines, IL-10 significantly contributes to both acute and chronic viral infection [2,3,15]. Viruses exploit the IL-10 induction pathway in order to dampen the adaptive immune responses against them and this eventually leads to the failure of viral clearance from the host and subsequent establishment of a chronic or persistent phase of infection. In particular, there are numerous reports regarding the association of IL-10 with chronic/persistent viral infection [15]. In previous reports of lymphocytic choriomeningitis virus (LCMV) infection, it was shown that IL-10 expression level alone was sufficient to explain LCMV clearance or persistence [3]. Recent reports comparing plasma IL-10 levels in hepatitis C virus (HCV)-infected and healthy individuals have shown that IL-10 levels in chronic HCV infection were markedly increased when compared to healthy individuals [2]. IL-10 has been shown to possess broad-spectrum anti-inflammatory activity which has been well demonstrated in various viral infections especially persistent infections. Thus, IL-10 is considered to be associated with the immunosuppression that is commonly found in chronic and persistent viral diseases. It may also inhibit viral elimination from the host. Therefore, IL-10 may be an important factor in the development and maintenance of chronic viral infection, as seen in PCV2 infection of pigs. In the present study, we evaluated the IL-10 expression in naturally occurring PCV2-infected lymphoid tissues and ascertained its cellular source especially looking at T and B cells and its co-localization with PCV2 antigen expressing cells.
Previous studies have shown that IL-10 expression is up-regulated in PCV2-associated PMWS at both systemic mRNA and secretory protein levels [6,8]. Results have shown that PCV2-infected pigs exhibit increased IL-10 mRNA expression only in the thymus, while mRNA levels were consistently decreased in most other lymphoid tissues such as inguinal and bronchial lymph nodes, and spleen [8]. Massive destruction of the thymic structure and depletion of normal resident thymocytes were also present in these animals [8]. However, contrasting reports indicated that IL-10 levels were not up-regulated at mRNA and protein levels in PCV2-infected cells in vitro [21]. From these studies, it seems that T cell rich lymphoid tissue, such as thymus, contains elevated IL-10 levels during PCV2 infection. PMWS is a multifactorial disease which is typically caused by multiple etiological agents in addition to PCV2 in field cases. Hence, it is thought that distribution of activated T cells in response to PCV2 infection may be influenced by the level of different pathogens distributed in different tissues. Thus, the distribution of activated T cells in response to PCV2 may be a source of variation in IL-10 levels. In addition, T regulatory cells were implicated in the induction of IL-10 in PMWS-affected pigs [12]. Above all, none of the previous studies determined the cellular distribution of IL-10 during naturally occurring PCV2 infection. With the objective of determining the distributing the cell populations that secrete IL-10 during PCV2 infection, we demonstrated in the present study that IL-10 expression occurs in both mandibular lymph node and spleen from pigs naturally affected by PCV2. Our results indicate that IL-10 is predominantly expressed in T-cell areas of the marginal zone of lymphoid follicles in mandibular lymph node and in splenic nodule, whereas it is rarely expressed by B-cells. The IL-10 expression in PCV2-infected lymphoid tissues was found to be significantly higher than that of uninfected control tissues (p<0.01). DL-IHC results showed that co-localization of IL-10+ cells and CD3+ T-cells was markedly increased in both mandibular lymph node (28%) and spleen (22.22%) when compared with that of IL-10+ cells and CD79a+ B-cells in mandibular lymph node (10.9%) and spleen (8.5%). Thus, 61% to 69% of the cells positive for IL-10 were neither T nor B cells. It is well known that macrophages and dendritic cells are strong inducers of IL-10 [11]. Unfortunately, we did not perform CD1 staining for dendritic cells in our tissue samples. Therefore, one cannot rule out the possibility of these remaining non-T and non-B cells being macrophages or dendritic cells. Most of the IL-10-expressing B cells in our study were located in the marginal zone, while very few B cells present in the germinal centers expressed IL-10. This result thus suggests that infiltrating naive B cells located in close approximation to PCV-infected cells in T cell rich areas could be vulnerable to IL-10 induction in response to PCV2 infection. These observations are consistent with IL-10 expression being mainly located in T cell rich areas rather than B cell rich areas. In contrast to these results, a previous report demonstrated that there was no upregulation of IL-10 mRNA expression in either lymph nodes or spleens of PMWS-affected pigs [8,21]. The variation in IL-10 expression in PCV2-infected pigs in field cases may also be explainable by the influence of various predisposing and etiological stresses observed in affected pigs. According to recent studies of IL-10 in PCV2 infection [7,8], as well as our own findings, it seems reasonable to assume that tissues with high levels of PCV2 antigen express much less IL-10 compared to tissues expressing higher levels of IL-10 that are found to be less or moderately infected with PCV2. Even though we did not classify our field cases as either acute or chronic infections, we assume that higher PCV2 antigen density is associated with acute infection and that lower antigen density is seen in chronic cases. If this is true, IL-10 may play a significant role during the chronic rather than the acute phase of PCV2 infection, illustrating its possible contribution to immunosuppression in chronic PCV2-infected pigs.
IL-10 detection in lymphoid tissues was characterized by strong and distinct cytoplasmic antigen deposition. Moreover, IL-10-positive cells were not colocalized with PCV2-positive cells. In the examination of the morphology of IL-10-positive cells, most of the cells did not exhibit typical macrophage morphology. This finding is consistent with the absence of co-localization of IL-10+ cells with PCV2+ cells.
Our current findings using the tissue samples from a small number of cases of natural PCV2 infection showed that the lymphoid tissues from pigs naturally affected by PCV2 infection exhibited elevated IL-10 expression compared to control tissues. The IL-10 producing cells were predominantly among T cell rich populations but were rarely present in B-cell rich areas. In addition, those IL-10 producing cells were rarely found to be macrophages. Moreover, PCV2-infected cells found in the tissues from naturally infected pigs rarely produced IL-10. These conclusions need to be further assessed with more studies with larger numbers of naturally or experimentally infected pigs and broader ranges of target tissues in order to definitively prove a direct or indirect role of IL-10 in PCV2 pathogenesis.
In summary, the lymphoid tissues from pigs naturally affected by PCV2 infection exhibited elevated IL-10 expression when compared to control tissues. In this study, IL-10 producing cells were mostly located in T cell rich populations and rarely found in macrophage and B-cell rich areas. In addition, PCV2-infected cells found in the tissues from naturally PCV2-infected pigs rarely produced IL-10.
Figures and Tables
Fig. 1
Immunohistochemical results showing expression of interukin (IL)-10 (A-C), CD3 (D), CD79a (E), and PCV2 antigens (F) in mandibular lymph nodes, spleen, and tonsil. (A) Mandibular lymph node; cytoplasmic staining of IL-10 antigens in marginal zone of lymphatic nodule. Scale bar = 140 µm. (B) Spleen; strong cytoplasmic staining of IL-10 antigen in splenic nodule. Scale bar = 56 µm. Inset: Higher magnification of IL-10 expression. (C) Tonsil; cytoplasmic staining of IL-10 antigen in and around tonsilar crypt. Scale bar = 56 µm. (D) Mandibular lymph node; strong immunohistochemical reaction to swine CD3, Scale bar = 140 µm. (E) Mandibular lymph node; strong immunohistochemical reaction to human CD79a. Scale bar = 140 µm. (F) Mandibular lymph node; presence of PCV2 antigen in the cytoplasm of macrophages and syncytial cells (arrows). Scale bar = 56 µm. GC: germinal center, PCN; paracortical nodule, RP: red pulp. (A), (B), (C) and (F) Alkaline phosphatase (red color) and hematoxylin stain. (D) and (E) Horse radish peroxidase (brown color) and hematoxylin stain.

Fig. 2
Double-labeling immunohistochemistry reaction to IL-10, CD3, CD79a, and PCV2 of mandibular lymph node and spleen. A: Mandibular lymph node; positive reaction of IL-10 and CD3 expression in the marginal zone. Scale bar = 140 µm. Inset: Higher magnification of marginal zone for IL-10 and CD3. (B) Spleen; strong positive reaction of IL-10 and CD3 expression in the marginal zone and red pulp. Scale bar = 140 µm. Inset: Higher magnification of marginal zone for IL-10 and CD3. (C) Mandibular lymph node; positive reaction of IL-10 expression in the marginal zone and CD79a in the germinal center. Scale bar = 140 µm. Inset: Higher magnification of marginal zone for IL-10 and CD79a. (D) Mandibular lymph node; positive reaction of both IL-10 and PCV2 antigens in marginal zone. Scale bar = 56 µm. GC: germinal center; PCN: paracortical nodule; RP: red pulp. (A), (B) and (C) Alkaline phosphatase (red color for IL-10), horse radish peroxidase (brown color for CD3) and hematoxylin stain. (D) Alkaline phosphatase (red color for PCV2), horse radish peroxidase (brown color for IL-10) and hematoxylin stain.
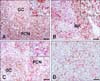
Table 1
The proportion of CD3 and CD79a positive cells to total interlukin (IL)-10 positive cells in two lymphoid organs of porcine circovirus type 2 (PCV2)-infected pigs by double-labeling immunohistochemistry (DL-IHC)

Acknowledgments
We wish to thank the valuable collaboration of Dr. S-Y Lee and Mrs. R-H Jang from the veterinary pathology service of Konkuk University. This study was supported in part by a grant from the Konkuk University Research Foundation 2008-2009. This study was also supported by the School of Veterinary Medicine and Biomedical Sciences, University of Nebraska-Lincoln, Lincoln, NE, USA.
References
1. Allan GM, McNeilly F, Kennedy S, Daft B, Clarke EG, Ellis JA, Haines DM, Meehan BM, Adair BM. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J Vet Diagn Invest. 1998. 10:3–10.

2. Barrett L, Gallant M, Howley C, Bowmer MI, Hirsch G, Peltekian K, Grant M. Enhanced IL-10 production in response to hepatitis C virus proteins by peripheral blood mononuclear cells from human immunodeficiency virus-monoinfected individuals. BMC Immunol. 2008. 9:28.

3. Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006. 12:1301–1309.

4. Chae C. Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet J. 2004. 168:41–49.

5. Chang HW, Jeng CR, Lin CM, Liu JJ, Chang CC, Tsai YC, Chia MY, Pang VF. The involvement of Fas/FasL interaction in porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus co-inoculation-associated lymphocyte apoptosis in vitro. Vet Microbiol. 2007. 122:72–82.

6. Chung HK, Chae C. Expression of interleukin-10 and interleukin-12 in piglets experimentally infected with porcine reproductive and respiratory syndrome virus (PRRSV). J Comp Pathol. 2003. 129:205–212.

7. Darwich L, Balasch M, Plana-Durán J, Segalés J, Domingo M, Mateu E. Cytokine profiles of peripheral blood mononuclear cells from pigs with postweaning multisystemic wasting syndrome in response to mitogen, superantigen or recall viral antigens. J Gen Virol. 2003. 84:3453–3457.

8. Darwich L, Pié S, Rovira A, Segalés J, Domingo M, Oswald IP, Mateu E. Cytokine mRNA expression profiles in lymphoid tissues of pigs naturally affected by postweaning multisystemic wasting syndrome. J Gen Virol. 2003. 84:2117–2125.

9. Darwich L, Segalés J, Resendes A, Balasch M, Plana-Durán J, Mateu E. Transient correlation between viremia levels and IL-10 expression in pigs subclinically infected with porcine circovirus type 2 (PCV2). Res Vet Sci. 2008. 84:194–198.

10. Faldyna M, Samankova P, Leva L, Cerny J, Oujezdska J, Rehakova Z, Sinkora J. Cross-reactive anti-human monoclonal antibodies as a tool for B-cell identification in dogs and pigs. Vet Immunol Immunopathol. 2007. 119:56–62.

11. Gómez-Laguna J, Salguero FJ, Barranco I, Pallarés FJ, Rodríguez-Gómez IM, Bernabé A, Carrasco L. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J Comp Pathol. 2010. 142:51–60.

12. Grierson SS, King DP, Tucker AW, Donadeu M, Mellencamp MA, Haverson K, Banks M, Bailey M. Ontogeny of systemic cellular immunity in the neonatal pig: correlation with the development of post-weaning multisystemic wasting syndrome. Vet Immunol Immunopathol. 2007. 119:254–268.

13. Kekarainen T, Montoya M, Mateu E, Segalés J. Porcine circovirus type 2-induced interleukin-10 modulates recall antigen responses. J Gen Virol. 2008. 89:760–765.

14. Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993. 11:165–190.

15. Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008. 226:205–218.

16. Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007. 19:591–615.

17. Quintana J, Segalés J, Rosell C, Calsamiglia M, Rodríguez-Arrioja GM, Chianini F, Folch JM, Maldonado J, Canal M, Plana-Durán J, Domingo M. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet Rec. 2001. 149:357–361.

18. Rosell C, Segalés J, Plana-Durán J, Balasch M, Rodríguez-Arrioja GM, Kennedy S, Allan GM, McNeilly F, Latimer KS, Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol. 1999. 120:59–78.

19. Segalés J, Domingo M, Chianini F, Majó N, Domínguez J, Darwich L, Mateu E. Immunosuppression in postweaning multisystemic wasting syndrome affected pigs. Vet Microbiol. 2004. 98:151–158.

20. Segalés J, Rosell C, Domingo M. Pathological findings associated with naturally acquired porcine circovirus type 2 associated disease. Vet Microbiol. 2004. 98:137–149.

21. Sipos W, Duvigneau JC, Willheim M, Schilcher F, Hartl RT, Hofbauer G, Exel B, Pietschmann P, Schmoll F. Systemic cytokine profile in feeder pigs suffering from natural postweaning multisystemic wasting syndrome (PMWS) as determined by semiquantitative RT-PCR and flow cytometric intracellular cytokine detection. Vet Immunol Immunopathol. 2004. 99:63–71.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download