Abstract
Severe acute respiratory syndrome (SARS) is a life-threatening disease for which accurate diagnosis is essential. Although many tools have been developed for the diagnosis of SARS, false-positive reactions in negative sera may occur because of cross-reactivity with other coronaviruses. We have raised polyclonal and monoclonal antibodies (Abs) using a recombinant form of the SARS virus nucleocapsid protein. Cross-reactivity of these anti-SARS Abs against human coronavirus (HCoV) 229E and HCoV OC43 were determined by Western blotting. The Abs produced reacted with recombinant SARS virus nucleocapsid protein, but not with HCoV 229E or HCoV OC43.
Severe acute respiratory syndrome (SARS) virus nucleocapsid (N) protein is abundantly released in the patients' blood during early infection [6], which suggested that the N protein is a suitable candidate for diagnostic applications. Detection of specific circulating Abs is potentially a highly sensitive and specific way to diagnose SARS. However, detection of SARS Abs from early-infected patients is difficult, because most patients experience IgG seroconversion on average 20 days after the onset of symptoms [5]. On the other hand, a highly conserved motif (FYYLGTGP: 111-118 a.a.), occurs in the N-terminal half of all coronavirus N proteins, and other conserved residues are reported to occur near this highly conserved motif [6]. The cross reactivity regions between SARS-coronavirus (CoV) and porcine CoVs were located in 120-208 a.a. of N protein [12]. Cross-reactivity between SARS-CoV N protein and anti-human CoV (HCoV) 229E Abs or anti-HCov OC43 Abs has been reported [14]. It is therefore necessary to develop an assay that shows no cross-reactivity with other CoVs, especially HCoVs, to detect SARS proteins. Such a test could be used for early detection and follow-up of patients during treatment. In this study, we raised polyclonal and monoclonal Abs using a recombinant SARS N protein to develop a specific diagnostic test for SARS.
The cDNA of SARS-CoV strain Hanoi provided by Dr. Kariwa of Hokkaido University was used as a template for PCR by Accupower premix (Bioneer, Korea). The full length of the SARS-CoV N gene was inserted into vector pET21a (Novagen, German). After transformation, E. coli strain Origami B (DE3) pLysS competent cells were induced using 1 mM IPTG (Invitrogen, USA) at 37℃ for 4 h. The expressed proteins were purified using the His-Bind kits (Novagen, German). Production of polyclonal and monoclonal Abs was performed as described by Shang et al. [7]. Human coronaviruses, HCoV 229E (ATCC, VR-730) and HCoV OC43 (ATCC, VR-1558), were infected to MRC-5 cells (Korean Cell Line Bank). Replication of these viruses was confirmed by RT-PCR of cell lysates infected with HCoV 229E and HCoV OC43. RT-PCR was performed as described in a previous study [10]. To examine whether Abs against recombinant N protein of the SARS virus react with other HCoVs, we performed Western blotting on recombinant SARS N protein or cell lysates infected with HCoV 229E and HCoV OC43.
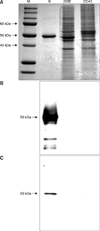
In our previous study, the antigenicity of recombinant of SARS-CoV N protein was checked with a mouse anti-SARS-CoV N protein monoclonal IgG2a (Zymed, USA), and convalescent SARS serum provided by the National Institute of Hygiene and Epidemiology in Vietnam [4]. We selected polyclonal and monoclonal Abs that showed the highest reactivity with the N protein in an ELISA. With these Abs, we determined cross-reactivity against cell lysates infected with HCoV 229E and HCoV OC43 by Western blotting. The viruses in these cells lysates were confirmed by RT-PCR (Fig. 1). Abs reacted with recombinant N protein, but did not react with HCoVs in cell lysates (Fig. 2). To determine the specificity of these Abs, cross reactivity with porcine epidemic diarrhea virus (coronavirus group I) and mouse hepatitis virus (coronavirus group II) were analyzed by Western blotting but showed no reaction (data not shown).
Coronaviruses are a group of large, enveloped, positive-sense, single-stranded RNA viruses that are known to associate with respiratory, enteric and neurological diseases in humans and domestic animals [2]. Many researchers have reported cross-reactivity with other HCoV when the diagnostic systems are based on SARS N protein [9,14]. It is therefore important to explore the possibility of developing a diagnostic test for SARS-CoV that does not show this cross-reactivity with the other HCoVs. Only two coronaviruses, HCoV 229E (Group I) and HCoV OC43 (Group II), have previously been known to cause illness in humans [2]. These coronaviruses are responsible for 10~35% of upper respiratory tract infections [9]. Another human coronaviruses, HCoV NL63 and Co HKU1, were reported in 2004 and 2005 [11,13]. As such, a SARS diagnostic system that cross-reacts with HCoVs could easily result in false-positive reactions. Previous researchers have tried to develop a monoclonal Ab against SARS N protein based ELISA. Some checked cross-reactivity with chicken CoV [3], HCoV OC43 [8] and various CoVs [1]. We need more Ab candidates for the diagnosis of SARS. In this study, we checked Abs cross-reactivity against SARS virus with HCoVs 229E and OC43, before developing a diagnostic system. Because the polyclonal and monoclonal Abs produced in this study did not react with HCoV 229E or HCoV OC43 in Western blotting, it could be possible to develop a specific diagnostic system to detect SARS-CoV in infected patients with theses Abs. Cross-reactivity with HCoV NL63 and Co HKU1, newly arising HCoVs, should be confirmed to strengthen the specificity of our Abs against SARS-CoV.
Figures and Tables
Fig. 1
RT-PCR of cell lysates infected with human coronavirus (HCoV) 229E and HCoV OC43. A: HCoV 229E specific RT-PCR, B: HCoV OC43 specific RT-PCR. The results of RT-PCR were consistent with virally infected MRC-5 cell. Lane M: 100 bp DNA ladder, Lane MRC-5: normal MRC-5 cell lysates, Lane 229E: HCoV 229E infected MRC-5 cell lysates, Lane OC43: HCoV OC43 infected MRC-5 cell lysates.
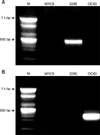
Fig. 2
Western blotting for detecting cross reactivity of polyclonal antibody (Ab) and monoclonal Ab with HCoVs 229E and OC43. A: SDS-PAGE, B: reacted with polyclonal Ab, C: reacted with monoclonal Ab. Purified recombinant N protein (Lanes N), HCoV 229E infected cell lysates (Lanes 229E) and HCoV OC43 infected cell lysates (Lanes OC43) were run in SDS PAGE 12% gels with molecular weight markers in Lane M.
Acknowledgments
We would like to thank the JungGyeom Cooperation for its assistance with monoclonal antibody production. This work was supported by grants-in-aid from the Korea Food and Drug Administration and the Korea Research Foundation (KRF-005-E00077). This work was also partially supported through the BK21 Program for Veterinary Science.
References
1. Che XY, Qiu LW, Pan YX, Wen K, Hao W, Zhang LY, Wang YD, Liao ZY, Hua X, Cheng VCC, Yuen KY. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol. 2004. 42:2629–2635.

2. Fields BN, Knipe DM, Howley PM, Griffin DE. Fields' Virology. 2001. Philadelphia: Lippincott Williams & Wilkins;1163–1203.
3. He Q, Du Q, Lau S, Manopo I, Lu L, Chan SW, Fenner BJ, Kwang J. Characterization of monoclonal antibody against SARS coronavirus nucleocapsid antigen and development of an antigen capture ELISA. J Virol Methods. 2005. 127:46–53.

4. Lee HK, Lee BH, Dutta NK, Seok SH, Baek MW, Lee HY, Kim DJ, Na YR, Noh KJ, Park SH, Kariwa H, Nakauchi M, Mai LQ, Heo SJ, Park JH. Detection of antibodies against SARS-coronavirus using recombinant truncated nucleocapsid proteins by ELISA. J Microbiol Biotechnol. 2008. 18:1717–1721.
5. Peiris JSM, Chu CM, Cheng VCC, Chan KS, Hung IFN, Poon LLM, Law KI, Tang BSF, Hon TYW, Chan CS, Chan KH, Ng JSC, Zheng BJ, Ng WL, Lai RWM, Guan Y, Yuen KY. members of the HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003. 361:1767–1772.

6. Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Peñaranda S, Bankamp B, Maher K, Chen MH, Tong S, Tamin A, Lowe L, Frace M, DeRisi JL, Chen Q, Wang D, Erdman DD, Peret TC, Burns C, Ksiazek TG, Rollin PE, Sanchez A, Liffick S, Holloway B, Limor J, McCaustland K, Olsen-Rasmussen M, Fouchier R, Günther S, Osterhaus AD, Drosten C, Pallansch MA, Anderson LJ, Bellini WJ. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003. 300:1394–1399.

7. Shang B, Wang XY, Yuan JW, Vabret A, Wu XD, Yang RF, Tian L, Ji YY, Deubel V, Sun B. Characterization and application of monoclonal antibodies against N Protein of SARS-coronavirus. Biochem Biophys Res Commun. 2005. 336:110–117.

8. Shin GC, Chung YS, Kim IS, Cho HW, Kang C. Preparation and characterization of a novel monoclonal antibody specific to severe acute respiratory syndrome-coronavirus nucleocapsid protein. Virus Res. 2006. 122:109–118.

9. Sizun J, Yu MW, Talbot PJ. Survival of human coronaviruses 229E and OC43 in suspension and after drying on surfaces: a possible source of hospital-acquired infections. J Hosp Infect. 2000. 46:55–60.

10. Vabret A, Mouthon F, Mourez T, Gouarin S, Petitjean J, Freymuth F. Direct diagnosis of human respiratory coronaviruses 229E and OC43 by the polymerase chain reaction. J Virol Methods. 2001. 97:59–66.

11. van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-vanDillen PM, Kaandorp J, Spaargaren J, Berkhout B. Identification of a new human coronavirus. Nat Med. 2004. 10:368–373.

12. Vlasova AN, Zhang X, Hasoksuz M, Nagesha HS, Haynes LM, Fang Y, Lu S, Saif LJ. Two-way antigenic cross-reactivity between severe acute respiratory syndrome coronavirus (SARS-CoV) and group 1 animal CoVs is mediated through an antigenic site in the N-terminal region of the SARS-CoV nucleoprotein. J Virol. 2007. 81:13365–13377.

13. Woo PCY, Lau SKP, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BHL, Poon RWS, Cai JJ, Luk WK, Poon LLM, Wong SSY, Guan Y, Peiris JSM, Yuen KY. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005. 79:884–895.

14. Woo PCY, Lau SKP, Wong BHL, Chan KH, Hui WT, Kwan GSW, Peiris JSM, Couch RB, Yuen KY. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polypeptide. J Clin Microbiol. 2004. 42:5885–5888.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download