Introduction
Calcium (Ca2+) elicits activation of complex intracellular signaling cascades and intracellular messengers. Cytosolic-free Ca2+ is crucial role in maintaining living organs and tissues, however, the mechanisms that couple membrane receptor activation to Ca2+ signaling, and the pathways of Ca2+ entry into the cytosol are only partly understood [6,28]. A 9-kilodalton cytosolic calcium-binding protein termed calbindin-D9k (CaBP-9k), which has two Ca2+ binding domains, belongs to a family of intracellular proteins that display high affinities for Ca2+ [8]. Full-length cDNA encoding human CaBP-9k has been cloned using reverse transcription-polymerase chain reaction (RT-PCR), which includes a coding region of 79 amino acids, a non-coding region comprising 57 5' nucleotides and 159 3' nucleotides, and a poly(A) tail, for a total length of 600 nucleotides [17]. Furthermore, the latter report revealed that this gene spans about 5.5-kb and is localized on the X-chromosome, consists of three exons, and carries four Alu repeats [18]. CaBP-9k is expressed in a variety of mammalian tissues, including tissues in the uterus, placenta, intestine, kidney, pituitary gland, and bone [1,25,26,33]. Research concerning the function of the calbindins suggests that calbindins participate in the process of Ca2+ transport across cellular barriers [38].
In the duodenum, the functional role of CaBP-9k appears to be involved in intestinal calcium absorption and its gene is regulated at the transcriptional or post-transcriptional level by 1, 25-dihydroxyvitamin D3 (1,25-(OH)2D3), a hormonal form of vitamin D [10,39]. They represent 2-3% of the total proteins in enterocytes (the major epithelial cell type of the duodenal mucosa), where active Ca2+ transport occurs [39]. In the kidney, CaBP-9k is expressed in the distal convoluted tubules, where it is thought to facilitate calcium re-absorption [30]. In addition, uterine CaBP-9k may be involved in controlling myometrial activity related with intracellular Ca2+ level [26], but its exact role is still unclear. Uterine CaBP-9k is mainly expressed in myometrial and endometrial stromal cells in nonpregnant rats, but not in the luminal epithelial cells, while CaBP-9k expression is detected predominantly in luminal uterine epithelial cells in pregnant rats [4,10,11,29]. Interestingly, uterine CaBP-9k is not regulated by vitamin D despite the presence of vitamin D receptors in this tissue. Rather, regulation of CaBP-9k may be under the control of sex steroid hormones [9,11,20-22].
The expression of the CaBP-9k gene has been explored in most mammalians except canines. The present study was undertaken to elucidate the expression of CaBP-9k mRNA in the uterus, duodenum, and kidney of beagles by RT-PCR and real-time PCR. In addition, the protein expression and localization of CaBP-9k were examined in these tissues by Western blot analysis and immunohistochemistry, respectively.
Materials and Methods
Experimental animals and treatments
The experiments were performed using two 2.5-year-old female beagle dogs. Both dogs were individually housed in a polycarbonate cage with alternating 12 h light/dark cycles in an environmentally controlled room (temperature: 23 ± 2℃ relative humidity: 50 ± 10%, frequent ventilation). During the acclimation period, they were fed with a commercial diet (Proplan; Nestle Purina Petcare, Korea) and tap water. To collect tissues, the dogs were euthanized and then the abdominal cavity was exposed by midline incision. The proximal duodenum, cortex of kidney and uterine horn were collected. All animal experimental procedures were approved by the Ethics Committee of the Chungbuk National University.
Total RNA extraction and RT-PCR
The collected duodenum, kidney and uterus were rapidly excised and washed in cold sterile saline (0.9% NaCl). Total RNA was prepared with TRIzol reagent (Invitrogen, USA) according to the manufacturer's protocol and the RNA concentration was determined by measurement at 260 nm. RT-PCR was performed and the obtained products were visualized by agarose gel electrophoresis. In brief, total RNA (1 µg) was reverse transcribed to first standard complementary DNA (cDNA) using mMLV reverse transcriptase (Invitrogen, USA) and random primers (9 mers; TaKaRa Bio, Japan). CaBP-9k and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as a housekeeping gene, were amplified in a 20 µL PCR reaction containing 1U i-StarTaq DNA polymerase (iNtRON Bio, Korea), 1.5 mM MgCl2, 2 mM dNTP, and 20 pmol CaBP-9k- or GAPDH-specific primers. The oligonucleotide sequences for CaBP-9k were 5'-AGT CTC AAG AAC TGA AG-3' (sense) and 5'-AAG AGG TCA TCT AGG GTG CT-3' (antisense). PCR reactions were denatured at 94℃ for 45 sec, annealed at 58℃ for 45 sec and extended at 72℃ for 45 sec. CaBP-9k and GAPDH were quantified after 27 cycles. PCR products (10 µL) were separated on a 2% agarose gel, stained with ethidium bromide, and photographed under ultraviolet illumination. Photographs were taken using a Gel Doc EQ (Bio-Rad, USA).
Real-time PCR analysis
Real-time PCR was performed in 20 µL reaction volumes containing 10 µL of SYBR premix Ex Taq (TaKaRa Bio, Japan) using a 7300 Real-time PCR system (Applied Biosystems, USA), following the manufacturer's recommendations. The oligonucleotide sequences for real-time PCR to detect CaBP-9k and GAPDH were identical as shown by RT-PCR. The relative expression levels of each gene were normalized to that of GAPDH and quantified using RQ software (Applied Biosystems, USA).
Western blot analysis
Protein was extracted using Proprep (iNtRON Bio, Korea), according to the manufacturer's protocol. Protein (180 µg per lane) was resolved by 12% sodium dodecyl sulfatepolyacrylamide gel electrophoresis and the resolved proteins were transferred to a polyvinylidene fluoride membrane (PerkinElmer Life Sciences, USA) The membrane was blocked 1 h with 5% bovine serum albumin (BSA) in Tris-buffed saline-Tween 20 (TBS-T). The membrane was incubated in primary antibodies diluted in 5% BSA for 5 h at room temperature. A 1 : 200 dilution of CaBP-9k Swant, Switzerland) and 1 : 500 dilution of β-Actin (Santa Cruz Biotechnology, USA) were used as primary antibody. A 1 : 3,000 dilution of horseradish peroxidase-conjugated anti-rabbit IgG (Santa Cruz Biotechnology, USA) was used as secondary antibody. Immunoreactive proteins were visualized using Western blot detection system (West-one; iNtRON Bio, Korea), according to the manufacturer's instructions. Signals were detected with Chemi Doc EQ (Bio-Rad, USA) and analyzed by Quantity One Program (Bio-Rad, USA).
Immunohistochemistry
Tissue localization of CaBP-9k protein was examined by immunohistochemistry. The duodenum, kidney and uterus tissue were individually fixed in 10% formalin overnight and then processed. Briefly, the tissues were dehydrated and embedded in paraffin, after which serial sections (7 µm) were cut with a microtome. Sections were deparaffinized in xylene and hydrated in descending grades of ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxidase in PBS-Tween 20 for 30 min, and then sections were incubated in 10% normal goat serum (NGS) for 2 h at room temperature to block nonspecific binding. Sections were incubated with a 1 : 200 dilution of polyclonal rabbit antibody specific to CaBP-9k (Swant, Switzerland) dissolved in 10% NGS and incubated at 4℃ overnight. After washing with PBS-T, the sections were incubated with a 1 : 500 dilution of biotinylated secondary antibody (anti-rabbit IgG; Vector Laboratories, USA) for 30 min at 37℃ and further incubated with ABC-Elite (PK-6101; Vector Laboratories, USA) for 30 min at 37℃. Diaminobenzidine (Sigma-Aldrich, USA) was used as a chromogen, and the sections were counterstained with hematoxylin followed by mounting with a coverslip.
Results
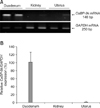
Expression of CaBP-9k mRNA in the duodenum, kidney and uterus
To examine the expression level of CaBP-9k mRNA, RT-PCR and real-time PCR were carried out. A 148 bp CaBP-9k transcript was observed in the canine duodenum. However, its expression in the kidney and uterus was undetectable as seen in Fig. 1. The expression of CaBP-9k mRNA as determined by RT-PCR (Fig. 1A) was consistent with the real-time PCR data expressed as a percentage of CaBP-9k/GAPDH mRNA (Fig. 1B).
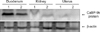
Expression of CaBP-9k protein in the duodenum, kidney and uterus
The expression of CaBP-9k protein was measured by Western blot analysis. In parallel with its mRNA level, the protein level of CaBP-9k was highly expressed in the duodenum, whereas its level was not detected in the canine kidney and uterus (Fig. 2).
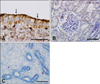
Localization of CaBP-9k protein in the duodenum
Immunohistochemistry for CaBP-9k protein in the duodenum revealed moderate staining along the surface of the villi, especially on the cytoplasm of the enterocytes, the major epithelial cells of the duodenal mucosa (Fig. 3).
Discussion
CaBP-9k is a small intracellular calcium-binding protein with two high-affinity Ca2+ binding domains [37]. It was originally isolated from the rat intestine as a vitamin D-dependent gene and was subsequently found to be expressed in mammalian intestine, kidney, placenta and uterus [2,12, 25]. The cytosolic calcium-binding proteins, CaBP-9k and CaBP-28k, play an important role in shuttling Ca2+ ions from the apical Ca2+ influx channels to the basolateral membrane [19,23], while the Na+/Ca2+ exchanger (NCX1) and plasma membrane Ca2+-ATPase 1b function in Ca2+ extrusion [31]. In the current paradigm of Ca2+ transport, CaBP-9k and CaBP-28k play an essential role in intestinal and renal calcium (re)absorption [13]. Expression of CaBP-9k has been explored in most mammalians except in a canine model [5-7]. Thus, in this study, we elucidated the expression of CaBP-9k mRNA and protein in the duodenum, kidney and uterus in a canine model.
RT-PCR and real-time PCR analysis revealed that expression level of CaBP-9k mRNA was abundant in the duodenum, but was undetectable in the kidney and uterus of the two beagle dogs under the present experimental conditions. In parallel with its mRNA level, the expression of CaBP-9k protein was detected solely in the duodenum.
There were differences in the regulation of CaBP-9k gene expression among different species [6,7]. For instance, CaBP-9k is expressed in the mouse kidney, but not in the rat kidney [24,32]. In parallel with the absence of CaBP-9k in the rat kidney, renal CaBP-9k was not observed in the two canines studied presently. Renal CaBP-9k is expressed in the distal convoluted tubule, where it is thought to facilitate calcium re-absorption [30]. Appropriately, we surmise that CaBP-9k in the kidney may be not essential for the proper function of this tissue, or that another and as yet unidentified intracellular calcium-binding protein exists. Although CaBP-9k was not detected in canine kidney and uterine tissues, other calcium-binding proteins such as CaBP-28k may play a role in Ca2+ transport in these tissues.
Uterine CaBP-9k expression has been detected in most mammalian species, including rat [3,11], mouse [29,34], cow [14], pig [16] and human [27]. But its expression levels in the uterus considerably differ among these species by estrous cycle or pregnancy. For example, the expression of CaBP-9k in the rat uterus is not detectable at diestrus, is increased at proestrus and reaches its zenith at estrus, thereafter decreasing as animals enter metestrus [6]. In addition, CaBP-9k is expressed mainly in the endometrial stroma and myometrial of the uterus in non-pregnant rats [3,11], whereas in pregnant rats it is expressed in uterine epithelial tissue [36].
Bovine CaBP-9k is expressed only in the luminal and glandular epithelium of the endometrium, and not in the myometrium or stromal cells of the endometrium in non-pregnant animals [14]. Therefore, a plausible reason why CaBP-9k was not detected in the canine uterus in the present study is that the two beagle dogs were in diestrous period. Alternatively, the protein may be completely absent, akin to the baboon uterus [15]. To identify the localization of the CaBP-9k protein in the duodenum, immunohistochemistry was performed. CaBP-9k was localized to enterocytes, the major epithelial cells of the duodenal mucosa [35], especially on the cytoplasm of the duodenocyte and along the surface of the villi.
In summary, the present study demonstrates the expression of CaBP-9k in the canine duodenum, but not kidney and uterine tissue. Whether the function of renal and uterine CaBP-9k is substituted by another intracellular calcium-binding protein or is not essential for proper functioning of these tissues remains to be determined. Further study is also needed to determine whether alteration of the estrous cycle is associated with CaBP-9k expression in the canine uterus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download