Abstract
A two-year-old male Pointer had been presented with anorexia, cachexia, and weight loss of 10-day duration. Upon physical examination, fever, lethargy, superficial lymph node enlargement, and tick infestation were noted. The only abnormality in CBC and serum chemistry analyses was mild hyperglobulinemia. Spleen was enlarged by radiography, and the lymph nodes showed neutrophilic lymphadenitis by cytological examination. A polymerase chain reaction test for babesiosis and commercial ELISA tests for Ehrlichia canis, heartworm, and Lyme disease was negative except for Lyme disease, which was verified by both an IFA-IgG test and a quantitative C6 assay. Doxycycline was administered for 2 weeks and the recovery was uneventful. Post-treatment C6 titer decreased to within normal limits.
Lyme disease is a tick-borne disease caused by Borrelia (B.) burgdorferi sensu lato. It affects humans and dogs worldwide, and has been reported in North America, Europe, Asia, Australia, South America, and Africa [3]. Vectors of B. burgdorferi sensu lato include various species of hard ticks of the Ixodes spp..
In Korea, B. afzelii, B. garinii, and B. valaisiana have been identified in ticks and several clinical cases of Lyme disease in humans have been reported [1,2,4,11]. However, there are no known occurrences in dogs. We report a possible case of Lyme borreliosis in a hunting dog evaluated by a commercial C6 ELISA kit, IFA, and a laboratory quantitative C6 assay.
A 2-year-old male Pointer dog was presented to Haemaru Referral Animal Hospital with signs of anorexia, cachexia, and weight loss of a 10-day duration. The body condition score decreased from 3/5 to 1/5 at presentation. The dog was born and raised in Kwangju, Kyonggi-do. It had been used in hunting, and was exposed to ticks. The hunting area covered Bongwha and Youngju, Kyongsangnam-do. Upon physical examination, a mild fever (39.7℃), lethargy, and enlargement of both superficial cervical lymph nodes were noted. Initial screening tests include CBC, serum chemistry, cytology of fine needle aspirates of enlarged lymph nodes, and abdominal radiography. Blood works and serum chemistry results were all within normal limits, except for mild hyperglobulinemia (4.6 g/dl, reference range 2.5-4.5 g/dl) (Table 1). The radiography showed that the spleen was enlarged (Fig. 1). Smears of lymph node aspirates revealed a predominant population of small lymphocytes with a lesser number of medium to large lymphocytes, plasma cells, neutrophils, and macrophages, consistent with neutrophilic lymphadenitis (Fig. 2). Based on this finding, increased serum globulin was considered to be associated with chronic inflammation, and lymphoproliferative neoplasia was ruled out. On the day of the first visit, infectious diseases were screened using a commercial ELISA kit (SNAP 3Dx; IDEXX, USA) for a heartworm (Ehrlichia canis) antigen, and a Lyme antibody (C6 antibody). All the tests were negative except for Lyme disease. On the same day, EDTA whole blood was also submitted to the College of Veterinary Medicine, Chonbuk National University, for PCR testing for E. canis and Babesia (the results were negative for both). To confirm the positive results of the ELISA kit for Lyme disease, the rest of the patient's serum was submitted to an IDEXX Reference Laboratory (USA) for a C6 titer assay and to Antech Diagnostics (USA) for a Lyme IgG assay. The C6 serum titer was 30 U/ml (positive ≥ 30), and the IgG titer was 1 : 256 (negative < 1 : 64). These results were highly suggestive of active borreliosis.
Treatment with doxycycline (10 mg/kg s.i.d, PO; Myung In Pharm, Korea) and supportive drugs (vitamin C, thiamine, serratiopeptidase and famotidine) was administered for 2 weeks. The dog responded well to treatment and the clinical signs disappeared. 3 weeks after the initiation of treatment, the lymph nodes had returned to normal size and serum globulin levels had decreased to 2.7 g/dl. Post-treatment C6 level was 10 U/ml.
C6 peptide is derived from the VlsE antigen, B. burgdorferi surface protein expressed when B. burgdorferi is transmitted to the dog but not expressed in the tick, in tissue culture, or in Lyme vaccines [5,7,8,10]. The sensitivities and specificities of this peptide-based ELISA are reportedly equivalent or superior to those of western blot assays alone or a combination of whole-cell based ELISA and western blot analysis [6,7,9]. The C6 antibody titer has been found to decrease post-treatment because production of the antibody to the C6 peptide may depend on the presence of a viable organism. For this reason, post treatment C6 levels may be used as an indicator of therapy outcome [6,7,10].
The patient in this report was presumptively diagnosed with Lyme borreliosis based on an increased C6 antibody titer. This diagnosis was confirmed by both a commercial Lyme antibody test kit and a laboratory quantitative C6 assay (IDEXX Reference Laboratory, USA) along with an increased IgG titer by IFA. Normal lymph nodes, resolution of the anorexia and fever, and decreased C6 antibody titer post treatment also supported our diagnosis. In a previous report, the median percent decline in C6 level relative to pretreatment values at 6 and 12 months were found to be 68.0% and 83.3%, respectively [6]. However, in this case, a PCR test using peripheral blood was unsuccessful. The negative PCR test may be attributed to the fact that it was done using a peripheral blood sample. Connective tissue, synovia, or skin samples near the tick bite are preferred for PCR because Borrelia organisms rarely spread hematogenously. The organism can also be isolated from the skin area for an extended period. In this case, there was tick infestation when it came to the animal hospital, so if a skin sample from near the tick bite site was used for PCR, positive results would have been possible. A skin biopsy sample from as close as possible to the tick-bite should be submitted for a reliable PCR test [3,12].
To the best of our knowledge, canine Lyme borreliosis has never been reported in Korea. This disease might be underestimated in dogs because B. burgdorferi sensu lato has been isolated in ticks and wild rodents in Korea [1,4,11]. Patients could also easily be overlooked due to good responses to therapy without a proper diagnosis. If a symptomatic dog has outdoor activity and a history of tick infestation, Lyme disease should be included in the differential diagnosis.
Figures and Tables
Fig. 1
Radiograph of the spleen. The abdomen was decreased by lack of fat. In the lateral view, soft tissue density structure (arrows) was identified in the ventral region of the middle abdomen, and this structure was considered to be splenomegaly based on the position and shape.
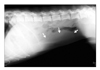
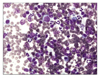
Fig. 2
Microphotograph of the enlarged superficial cervical lymph node. Note the mixed population of lymphocytes, neutrophils, and plasma cells with a predominance of small lymphocytes. Some of the cells were lysed in the background. Wright stain, ×400.
References
1. Chae JS, Yu do H, Shringi S, Klein TA, Kim HC, Chong ST, Lee IY, Foley J. Microbial pathogens in ticks, rodents and a shrew in northern Gyeonggi-do near the DMZ, Korea. J Vet Sci. 2008. 9:285–293.

2. Choi YJ, Han SH, Park JM, Lee KM, Lee EM, Lee SH, Song HJ, Koh YS, Lee KW, Jang WJ, Park KH. First molecular detection of Borrelia afzelii in clinical samples in Korea. Microbiol Immunol. 2007. 51:1201–1207.

3. Hartmann K, Greene CE. Ettinger SJ, Feldman EC, editors. Diseases caused by systemic bacterial infections. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 2005. 6th ed. St. Louis: Elsevier;619–625.
4. Lee SH, Kim BJ, Kim JH, Park KH, Kim SJ, Kook YH. Differentiation of Borrelia burgdorferi sensu lato on the basis of RNA polymerase gene (rpoB) sequences. J Clin Microbiol. 2000. 38:2557–2562.

5. Levy S, O'Connor TP, Hanscom JL, Shields P. Utility of an in-office C6 ELISA test kit for determination of infection status of dogs naturally exposed to Borrelia burgdorferi. Vet Ther. 2002. 3:308–315.
6. Levy SA, O'Connor TP, Hanscom JL, Shields P, Lorentzen L, Dimarco AA. Quantitative measurement of C6 antibody following antibiotic treatment of Borrelia burgdorferi antibody-positive nonclinical dogs. Clin Vaccine Immunol. 2008. 15:115–119.

7. Liang FT, Jacobson RH, Straubinger RK, Grooters A, Philipp MT. Characterization of a Borrelia burgdorferi VlsE invariable region useful in canine Lyme disease serodiagnosis by enzyme-linked immunosorbent assay. J Clin Microbiol. 2000. 38:4160–4166.

8. Littman MP, Goldstein RE, Labato MA, Lappin MR, Moore GE. ACVIM small animal consensus statement on Lyme disease in dogs: diagnosis, treatment, and prevention. J Vet Intern Med. 2006. 20:422–434.

9. Mogilyansky E, Loa CC, Adelson ME, Mordechai E, Tilton RC. Comparison of Western immunoblotting and the C6 Lyme antibody test for laboratory detection of Lyme disease. Clin Diagn Lab Immunol. 2004. 11:924–929.

10. O'Connor TP, Esty KJ, Hanscom JL, Shields P, Philipp MT. Dogs vaccinated with common Lyme disease vaccines do not respond to IR6, the conserved immunodominant region of the VlsE surface protein of Borrelia burgdorferi. Clin Diagn Lab Immunol. 2004. 11:458–462.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download