Abstract
Discovery of Helicobacter (H.) pylori has led to a fundamental change in our understanding of gastric diseases in humans. Previous studies have found various Helicobacter spp. in dogs and cats, and pets have been questioned as a zoonotic carrier. The present study surveyed the Helicobacter infections and investigated the presence of H. felis and H. pylori infections in domestic and feral cats in Korea. Sixty-four domestic cats and 101 feral cats were selected from an animal shelter. Saliva and feces were evaluated by Helicobacter genus-specific polymerase chain reaction (PCR). Genus-specific PCR positive samples were further evaluated for H. felis and H. pylori using specific primer pairs. Thirty-six of 64 (56.3%) samples from domestic cats and 92 of 101 (91.1%) samples from feral cats were PCR positive; the positive rate of feces samples was higher than that of saliva samples in both groups. H. felis and H. pylori species-specific PCR was uniformly negative. The prevalence of Helicobacter spp. in feral cats was approximately two-fold higher than that of domestic cats. The fecal-oral route may be more a common transmission route not only between cats but also in humans.
Since the discovery of Helicobacter (H.) pylori [28], gastritis has been studied from a whole new perspective. To date, spiral bacteria other than H. pylori found in stomach of humans, animals, dogs and cats have been considered a potential reservoir of zoonosis [7-9,13,17,19,20,31,32,36,41,44]. Two gastric Helicobacters, H. heilmannii and H. felis, are mostly associated with human gastric disease [14]. Nevertheless, eight other enterohepatic Helicobacters (H. canis, H. pullorum, H. cinaedi, H. fennelliae, H. canadensis, H. winghamensis, H. westmaedi, and H. rappini) have been isolated from humans [10].
In humans, Helicobacter spp. infections are associated with gastrointestinal diseases, cancers, and the immunocompromised. In dogs and cats, however, clinically healthy hosts are typically found. While H. felis is implicated as a potential pathogen in humans, many other species are still under research [14].
The route of transmission of Helicobacter spp. is uncertain, but is known to spread by direct contact. Oral-oral, gastro-oral, and fecal-oral routes are all possible [2]. Iatrogenic H. pylori infection transmitted by the endoscope or by contact with gastric fluid also has been reported [43].
H. pylori infection is predominant in the developing world, and low socio-economic status is associated with increased prevalence of the infection [27]. Fecal contamination of common sources including water [16] and soil [15] has been implicated in spread of the infection. This is supported by the findings that H. pylori infection rates are higher in developing countries, where untreated water and inadequately prepared vegetables contaminated with soil are common [4,15]. In animals, the DNA of Helicobacter spp. has been detected from sources other than gastric tissues, which include vomitus and saliva [37], dental plaques [37], and feces [18].
In Korea, Helicobacter spp. has been studied in many animals, for example, dogs [1,17,18,30,33,34], cats [17,22], pigs [35], mice [21], and Mongolian gerbils [24,25]. The DNA of several species of Helicobacter has been detected. 'H. heilmannii', formerly named Gastrospirillum hominis in humans [29], is the most predominant species known in cats [5,7,17,31,36,41]. H. felis [5,20,32,36,41] and H. canis [8,9] are also detected in cats. H. pylori was isolated from a group of cats from a commercial vendor of research animals [11,12], and the bacterial DNA has been detected in bile of cats [3]. In studies where specific pathogen free cats were experimentally inoculated with H. pylori or H. felis, the bacteria induced mild gastritis associated with lymphoid follicles, with no gastric erosions or ulcers evident during upper gastrointestinal endoscopy or at necropsy [38,39].
The present study was surveyed the prevalence of Helicobacter infection and the specific presence of H. felis and H. pylori infection, as a means of clarifying the possible role of domestic and feral cats in Korea as a zoonotic source.
Saliva and feces samples of 165 domestic and feral cats were obtained. Cats were grouped by environmental criteria; domestic cats were those that were almost exclusively kept indoors and feral cats being those that had been captured roaming wild in suburban areas. In Korea, government policy dictates that overpopulating feral cats are euthanized to preserve the wild life in suburban forests. During weekly visits to an animal shelter operated by the Korea Animal Rescue and Management Association, saliva and feces feral cats (n = 101; 55 females, 46 males) were obtained. Ages and health status of the cats were not ascertained. Domestic cats (n = 64; 28 females, 36 males) were either admitted to the Veterinary Medical Teaching Hospital of Seoul National University (Korea) or were the pets of staff members. The cats had an average age of 3.1 years (range 3 months to 12 years). Twenty-three cats were healthy and 41 were clinically ill; of the latter, the clinical signs varied from simple anorexia to hepatic lipidosis, feline lower urinary tract disease, renal failure, diabetes mellitus, and lymphoma. Feces and saliva samples taken from each cat by swabbing with sterilized cotton swabs were merged in 500 µl of autoclaved phosphate buffered saline. DNA was extracted from 20 µl of each sample using DNeasy Tissue Kit (Qiagen, USA). The DNA samples eluted 100-200 µl were stored at -20℃ until required.
Helicobacter 16S rRNA gene was amplified from each DNA sample using c97 and c98 primers (Table 1) [10]. The final reaction volume of 25 µl contained 2 µl of DNA sample, 12.5 pmole of each primer, ×1 PCR buffer (Takara Bio, Korea), 200 µM of deoxyribonucleoside triphophates mixture (Takara Bio, Korea), and 0.75 U of recombinant Taq DNA polymerase (Takara Bio, Korea). The PCR cycle was 94℃ for 2.5 min followed by 40 cycles of denaturation at 94℃, annealing at 50℃, extension at 72℃ for 1 min each, and a final extension at 72℃ for 15 min [17]. PCR was performed using a PC808 programmed temperature control system (Astec, Japan). PCR products were electrophoresed on ethidium bromide stained 1.5% w/v agarose gels in ×0.5 TBE buffer. The separated products were visualized on ultraviolet light illuminator. PCR sensitivity and specificity of fecal samples has been previously evaluated [18].
H. pylori and H. felis specific PCR was performed with primers (Table 1) that amplify the urease B gene of H. pylori and H. felis [31]. Two microliters of each DNA sample was added to a reaction mixture containing 12.5 pmole of each primer, ×1 PCR buffer (Takara Bio, Korea), 200 µM of deoxyribonucleoside triphophates mixture (Takara Bio, Korea), and 0.75 U of recombinant Taq DNA polymerase (Takara Bio, Korea) to produce a total volume of 25 µl. For H. pylori specific PCR, samples were heated to 95℃ for 5 min and 57℃ for 5 min once, followed by 35 cycles of extension at 72℃ for 1 min, denaturation at 94℃ for 1 min, annealing at 72℃ for 2 min, and a final extension at 72℃ for 10 min [41]. The positive control (isolates purchased from the American Type Culture Collection; ATCC, USA) and the negative control (sterile distilled water) were carried out with every PCR. For H. felis specific PCR, samples were heated to 94℃ for 2.5 min once, followed by 40 cycles of denaturation at 94℃, annealing at 47℃, extension at 72℃ for 1 min each, with a final extension at 72℃ for 15 min. The positive control (H. felis ATCC 49179) and the negative control (sterile distilled water) were carried out with every PCR. PCR products were electrophoresed on ethidium bromide stained 1.5% w/v agarose gels in ×0.5 TBE buffer. The separated PCR products were visualized using an ultraviolet light illuminator.
A specific sized PCR product was extracted using a MEGAquick-spin gel extraction kit (Intron, Korea) to confirm the identity of the target gene PCR product. Purified PCR products were analyzed using an ABI 3100 automatic sequence analyzer (Applied Biosystems, USA).
On Helicobacter genus-specific PCR for 16s rRNA gene, 36 (56.3%) from 64 domestic cats were positive, and 92 (91.1%) from 101 feral cats were positive on either saliva or feces samples (Fig. 1). In domestic cats, 17 (26.6%) saliva samples and 29 (45.3%) feces samples were positive. Infection rates were higher in feral cats with 47 (46.5%) saliva samples and 85 (84.2%) feces samples being positive (Table 2). Among the 64 domestic cats for which the clinical status was known, 36 (56.3%) were positive for Helicobacter spp. infection. Clinically ill cats had a Helicobacter spp. infection rate of 63.4% (26/41), compared to 43.5% (10/23) of healthy cats, which was not statistically significant. Ill cats were not especially prone to gastrointestinal diseases, and their diagnoses mainly involved anorexia with or without hepatic lipidosis, feline urologic syndrome, diabetes mellitus, renal failure, feline infectious peritonitis, lymphoma, and otitis.
Species-specific PCR was performed on 17 saliva samples and 29 feces samples from the domestic cats and 47 saliva samples and 85 feces samples from the feral cats, which showed a positive result on genus-specific PCR. In H. felis specific PCR, which amplified a 1,200 bp fragment in the positive control (ATCC 49179), none of the samples were positive (Fig. 2). Also, no samples were positive on H. pylori specific PCR, which revealed a 1,700 bp fragment on the positive control (SS1 strain) (Fig. 3).
Direct sequencing of the PCR product of the specific 400 bp band confirmed the amplified DNA fragments to be from the genus Helicobacter. Direct sequencing of the genus specific PCR product was performed on two randomly selected feces samples. One of the sequencing results was 100% identical with H. canis 16S rRNA gene. It displayed 99% similarity to the sequence of Helicobacter spp. 'feline isolate' 16S rRNA, H. canis strain Lausanne 16S rRNA, and H. canis NCTC 12220 16S rRNA. The other sequencing result resulted in 97% homology to H. canis strain MIT 51402, H. canis strain ATCC 51401, H. cholecystus strain ATCC 700242, and H. bilis strain FL56.
The prevalence of Helicobacter spp. between 64 domestic and 101 feral cats in Korea was compared in this study. Prevalence of Helicobacter spp. in either saliva or feces samples was 91.1% in feral cats and 56.3% in domestic cats suggesting that feral cats under shelter environments are at greater risk of Helicobacter spp. infection. The precise route of transmission of Helicobacter spp. is yet unknown, but other than fecal-oral or oral-oral routes, gastric juice after vomiting as the natural route of transmission is considered [2]. This gastro-oral hypothesis seems to be convincing considering the fact that the infection is typically acquired in early childhood in humans, specifically with epidemiologic vomiting and childhood overcrowding [2,23]. Moreover, since infection is predominant in developing countries and among intimate familial members [26], it seems likely that the cats in shelter environments are prone to Helicobacter spp. infection. Positive infection rates were higher on feces samples in both domestic and feral cats (46.5% and 84.2%, respectively) than on saliva samples (26.6% and 45.3%, respectively). This may suggest that under natural circumstances fecal-oral transmission is more likely than oral-oral transmission among cats. In a previous study, only fecal contact remained as a significant risk factor in an indirect study by questioning, and the seroprevalence for H. pylori increased significantly with age [6]. Nevertheless, healthy adult cats vomit naturally on occasion to spit out hairballs, which makes the gastro-oral route conceivable. The incidence of positive outcome between saliva and feces samples were random, meaning positive feces samples did not always have positive saliva samples, nor did positive saliva samples not always have positive feces samples.
Clinical signs were unknown in feral cats, and domestic cats did not have apparent gastrointestinal signs. Forty-one domestic cats admitted to the hospital for sickness mostly had systemic diseases, and gastrointestinal signs were of simple anorexia coupled with stress or secondary mucosal bleeding due to azotemia. In clinically healthy domestic cats the rate of Helicobacter spp. infection was 43.5% (10/23) and in clinically ill domestic cats the rate was 63.4% (26/41). Correlation between the infection rate and the clinical illness including gastrointestinal signs was not confirmative in this study due to the small number of cats involved. Further study of a larger cat population would be needed.
In species-specific studies, neither H. felis nor H. pylori were found. This is consistent with the results of some previous studies. A Swiss study of 58 cats reported that no amplification of H. felis or H. pylori were detected in PCR [31]. However, other studies that utilized PCR for examination of gastric biopsy samples reported H. felis was in four of 17 [41], two of 21 [17], and one of 15 [5] cats, and one of 10 cheetahs [42]. Although the numbers of cats in the present study was higher than in previous studies, not a single H. felis positive sample was evident. Presently, direct sequencing of two 16S rRNA gene-specific PCR products was conducted from purified isolates of genus-specific PCR. One of the two products displayed 100% similarity to a H. canis 16S rRNA sequence of 336 base pairs. The 16S rRNA sequences of H. felis and those of H. bizzozeronii and H. salomonis display 98.2-100% similarity [19], and H. canis differs by 8.1-10.1% from these species [40]. This implies that there is a significant genetic difference within the 16S rRNA gene of these Helicobacter species. H. canis also has been reported in cats in the United States [8,9].
In Korea, Helicobacter spp. studies in cats [17,22] have been fewer and not perceived as urgent a public health issue as similar studies conducted in dogs [1,17,30,33,34]. However, given the burgeoning population of domestic cats in Korea, and the likelihood that many of these cats are kept indoors in close contact with adults and children, careful study of the zoonotic potential of cats is warranted. While cats have not been regarded as a potential zoonotic threat for Helicobacter infections, the results of this study show prompt a re-examination of that view. It is suggested that care be taken especially when handling feces of domestic cats.
Figures and Tables
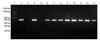 | Fig. 1PCR amplication of Helicobacter (H.) spp. genus-specific 16S rRNA gene. DNA molecular weight standard marker (Lane 1), H. felis positive control (ATCC 49179) of DNA product at 400 bps (Lane 2), negative control (Lane 3), feces of feral cats no.92-101 (Lanes 4-13) are shown. |
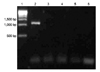 | Fig. 2PCR amplication of Helicobacter (H.) felis urease B gene fragment. DNA molecular weight standard marker (Lane 1), H. felis positive control (ATCC 49179) of DNA product at 1,150 bps (Lane 2), negative control (Lane 3), feces of domestic cats no. 12, 14, 16 (Lanes 4-6) are shown. All saliva and feces samples of positive Helicobacter genus-specific PCR were H. felis negative. |
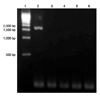 | Fig. 3PCR amplication of Helicobacter (H.) pylori urease B gene fragment. DNA molecular weight standard marker (Lane 1), H. pylori positive control of DNA product at 1,707 bps (Lane 2), negative control (Lane 3), feces of feral cats no.71-73 (Lane 4-6) are shown. All saliva and feces samples of positive Helicobacter genus-specific PCR were H. pylori negative. |
Acknowledgments
This work was supported by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University and Korean Research Foundation Grant (KRF-005-E00078).
References
1. An JH, Nam HW, Han JH, Kim D. The detection of Helicobacter-like organisms in dogs. Korean J Vet Clin Med. 1999. 16:281–288.
2. Axon ATR. Is Helicobacter pylori transmitted by the gastro-oral route? Aliment Pharmacol Ther. 1995. 9:585–588.
3. Boomkens SY, Kusters JG, Hoffmann G, Pot RGJ, Spee B, Penning LC, Egberink HF, Van den Ingh TS, Rothuizen J. Detection of Helicobacter pylori in bile of cats. FEMS Immunol Med Microbiol. 2004. 42:307–311.
4. Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000. 22:283–297.
5. Chisholm SA, Owen RJ. Development and application of a novel screening PCR assay for direct detection of 'Helicobacter heilmannii'-like organisms in human gastric biopsies in Southeast England. Diagn Microbiol Infect Dis. 2003. 46:1–7.

6. De Schryver A, Van Winckel M, Cornelis K, Moens G, Devlies G, De Backer G. Helicobacter pylori infection: further evidence for the role of feco-oral transmission. Helicobacter. 2006. 11:523–528.

7. Dieterich C, Wiesel P, Neiger R, Blum A, Corthésy-Theulaz I. Presence of multiple "Helicobacter heilmannii" strains in an individual suffering from ulcers and in his two cats. J Clin Microbiol. 1998. 36:1366–1370.

8. Foley JE, Marks SL, Munson L, Melli A, Dewhirst FE, Yu S, Shen Z, Fox JG. Isolation of Helicobacter canis from a colony of bengal cats with endemic diarrhea. J Clin Microbiol. 1999. 37:3271–3275.

9. Foley JE, Solnick JV, Lapointe JM, Jang S, Pedersen NC. Identification of a novel enteric Helicobacter species in a kitten with severe diarrhea. J Clin Microbiol. 1998. 36:908–912.

10. Fox JG. The non-H pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut. 2002. 50:273–283.

11. Handt LK, Fox JG, Dewhirst FE, Fraser GJ, Paster BJ, Yan LL, Rozmiarek H, Rufo R, Stalis IH. Helicobacter pylori isolated from the domestic cat: public health implications. Infect Immun. 1994. 62:2367–2374.

12. Handt LK, Fox JG, Stalis IH, Rufo R, Lee G, Linn J, LI X, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995. 33:2280–2289.

13. Hänninen ML, Happonen I, Saari S, Jalava K. Culture and characteristics of Helicobacter bizzozeronii, a new canine gastric Helicobacter sp. Int J Syst Bacteriol. 1996. 46:160–166.
14. Heilmann KL, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991. 32:137–140.

15. Hopkins RJ, Vial PA, Ferreccio C, Ovalle J, Prado P, Sotomayor V, Russell RG, Wasserman SS, Morris JG Jr. Seroprevalence of Helicobacter pylori in Chile: vegetables may serve as one route of transmission. J Infect Dis. 1993. 168:222–226.

16. Hulten K, Han SW, Enroth H, Klein PD, Opekun AR, Gilman RH, Evans DG, Engstrand L, Graham DY, El-Zaatari FA. Helicobacter pylori in the drinking water in Peru. Gastroenterology. 1996. 110:1031–1035.
17. Hwang CY, Han HR, Youn HY. Prevalence and clinical characterization of gastric Helicobacter species infection of dogs and cats in Korea. J Vet Sci. 2002. 3:123–133.

18. Hwang CY, Youn HY, Han HR. Development of noninvasive fecal PCR assay for detecting the Helicobacter species infection in dogs. J Vet Clin. 2002. 19:295–298.
19. Jalava K, Kaartinen M, Utriainen M, Happonen I, Hanninen ML. Helicobacter salomonis sp. nov., a canine gastric Helicobacter sp. related to Helicobacter felis and Helicobacter bizzozeronii. Int J Syst Bacteriol. 1997. 47:975–982.

20. Jalava K, ON SL, Vandamme PA, Happonen I, Sukura A, Hänninen ML. Isolation and identification of Helicobacter spp. from canine and feline gastric mucosa. Appl Environ Microbiol. 1998. 64:3998–4006.

21. Kim BH, Won YS, Lee CH, Hyun BH, Kim DY, Choi YK. Inflammatory large bowel disease due to Helicobacter hepaticus infection in BALB/cA-Hfh11nu mice. Korean J Lab Anim Sci. 1998. 18:143–146.
22. Kim SK, Cho SJ, Kim O. Detection and identification of secreting Helicobacter species from cats. Lab Anim Res. 2006. 22:243–247.
23. Kivi M, Tindberg Y. Helicobacter pylori occurrence and transmission: a family affair? Scand J Infect Dis. 2006. 38:407–417.
24. Lee JU, Jung K, Kim O. Absence of vertical transmission of Helicobacter pylori in an experimental murine model. J Vet Sci. 2006. 7:225–228.

25. Lee JU, Kim O. Natural maternal transmission of H. pylori in Mongolian gerbils. World J Gastroenterol. 2006. 12:5663–5667.
26. Magalhães Queiroz DM, Luzza F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2006. 11 Suppl 1:1–5.
27. Malaty HM, Kim JG, Kim SD, Graham DY. Prevalence of Helicobacter pylori infection in Korean children: inverse relation to socioeconomic status despite a uniformly high prevalence in adults. Am J Epidemiol. 1996. 143:257–262.

28. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984. 1:1311–1315.

29. McNulty CAM, Dent JC, Curry A, Uff JS, Ford GA, Gear MW, Wilkinson SP. New spiral bacterium in gastric mucosa. J Clin Pathol. 1989. 42:585–591.

30. Nam HW, Kim D. Prevalence of Helicobacter species infection in dogs. Korean J Vet Res. 2000. 40:747–753.
31. Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthésy-Theulaz I, Halter F, Lauterburg B, Schmassmann A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998. 36:634–637.

32. Norris CR, Marks SL, Eaton KA, Torabian SZ, Munn RJ, Solnick JV. Healthy cats are commonly colonized with "Helicobacter heilmannii" that is associated with minimal gastritis. J Clin Microbiol. 1999. 37:189–194.

33. Park JH, Lee BJ, Kim CK, Park TK, Park JH, Kim CH, Li GX, Lee YS. Pathological examination of stomachs from beagle dogs spontaneously infected with Gastrospirillum sp. Korean J Lab Anim Sci. 1998. 14:121–126.
34. Park JH, Park HM, Seok SH, Cho SA, Lee HY, Kim DJ, Park JH. Prevalence and pathological characteristics of Helicobacter spp. in gastric mucosa of domestic pet dogs. Korean J Lab Anim Sci. 2002. 18:120–124.
35. Park JH, Seok SH, Cho SA, Baek MW, Lee HY, Kim DJ, Park JH. The high prevalence of Helicobacter sp. in porcine pyloric mucosa and its histopathological and molecular characteristics. Vet Microbiol. 2004. 104:219–225.

36. Priestnall SL, Wiinberg B, Spohr A, Neuhaus B, Kuffer M, Wiedmann M, Simpson KW. Evaluation of "Helicobacter heilmannii" subtypes in the gastric mucosas of cats and dogs. J Clin Microbiol. 2004. 42:2144–2151.

37. Recordati C, Gualdi V, Tosi S, Facchini RV, Pengo G, Luini M, Simpson KW, Scanziani E. Detection of Helicobacter spp. DNA in the oral cavity of dogs. Vet Microbiol. 2007. 119:346–351.
38. Simpson KW, Strauss-Ayali D, Scanziani E, Straubinger RK, McDonough PL, Straubinger AF, Chang YF, Domeneghini C, Arebi N, Calam J. Helicobacter felis infection is associated with lymphoid follicular hyperplasia and mild gastritis but normal gastric secretory function in cats. Infect Immun. 2000. 68:779–790.

39. Simpson KW, Strauss-Ayali D, Straubinger RK, Scanziani E, McDonough PL, Straubinger AF, Chang YF, Esteves MI, Fox JG, Domeneghini C, Arebi N, Calam J. Helicobacter pylori infection in the cat: Evaluation of gastric colonization, inflammation and function. Helicobacter. 2001. 6:1–14.

40. Solnick JV, Schauer DB. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin Microbiol Rev. 2001. 14:59–97.

41. Strauss-Ayali D, Scanziani E, Deng D, Simpson KW. Helicobacter spp. infection in cats: evaluation of the humoral immune response and prevalence of gastric Helicobacter spp. Vet Microbiol. 2001. 79:253–265.

42. Terio KA, Munson L, Marker L, Aldridge BM, Solnick JV. Comparison of Helicobacter spp. in Cheetahs (Acinonyx jubatus) with and without gastritis. J Clin Microbiol. 2005. 43:229–234.

43. Tytgat GN. Endoscopic transmission of Helicobacter pylori. Aliment Pharmacol Ther. 1995. 9:Suppl 2. 105–110.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download