Abstract
In this case report, we present a mock-transduced bone marrow (BM) transplantation in a mouse, which was found moribund and autopsied to evaluate pathogenesis. Macroscopically, red discoloration of systemic organs was observed. Hematological values revealed a decrease in white blood cells, red blood cells, hematocrit, hemoglobin, and platelets, but an increase in reticulocytes. In BM cytology, hematopoietic cell lines were severely depleted. Histopathologically, hemorrhage in the cerebellar parenchyma, hemosiderin deposition and hemorrhage in the heart, necrosis and telangiectasia in liver, pulmonary parenchymal cysts, spermatogenic germ cells necrosis, atrophy and hemorrhage in testis, oligospermia and hemorrhage in the epididymis, and atrophy of BM, thymus and spleen were observed. In conclusion, autoimmune-like complications such as hematological value change, BM dysplasia and systemic hemorrhage appear to be the lethal cause of the mouse transplanted with mock-transduced BM.
Gene therapy with allogeneic or autologous bone marrow (BM) cells transduced with the normal gene sequence expressing the missing protein (s) comprises an attractive treatment for inherited hematological, non-hematological, and immunological defects [3,10]. Some viral vectors such as retroviral gene transfer [13] have been employed to transfer genes as well as BM transplantation (BMT) by many investigators [7]. Autoimmune-like complication after BMT leads morphological and functional changes of target tissues [9]. Some previous studies demonstrated that central nervous system (CNS), thymus, skin, liver, gastrointestinal tract, gonads, and heart are targeted in autoimmune-like complication [1,4,5,16]. However, reports dealing with the pathological features of BMT related-toxicity are limited.
In the present study, we report the histopathological features, hematological values, and BM cytology of a mouse transplanted with mock-transduced bone marrow, which was found moribund.
The carcinogenic effects of BMT with gene transduced BM via retroviral vector in a C57BL/6 male mouse was presented. The mouse was irradiated at 7.5 Gy from a Cs137 source. Transplanted BM cells were originally enriched hematopoietic stem cells isolated from the C57BL/6 male mouse and were transfected with the mock viral vector (empty particle) without any transgene. The tranduced BM cells were administered by tail vein injection at a dose of 35 × 106 cells/kg body weight. Total experimental period was 53 days, but the mouse was found moribund 19 days after BMT and autopsied to evaluate its pathogenesis. Selected hematology, histopathology, and bone marrow cytology measures were evaluated.
Hematological values (Table 1) revealed a decrease in the white blood cells (WBC), red blood cells (RBC), hematocrit, hemoglobin, and platelets, but an increase in reticulocytes.
In BM cytology, a small number of erythroid cells, plasma cells, macrophages, lymphoblasts, lymphocytes etc, were observed, but myeloid cells were almost completely depleted (Fig. 1, Table 2).
In the autopsy findings, red discoloration of subcutaneous, cerebellum, lymph nodes (submandibular and mesenteric), testis, epididymis and gastrointestinal tract were observed. Enlargement of the gall bladder was also noted.
Histopathologically, a severe hemorrhage was seen in cerebellar parenchyma (Fig. 2A). Pulmonary parenchymal cysts were also observed in the lung (Fig. 2B). Hemosiderin deposition, hemorrhage in the heart (Fig. 2C), hepatocellular necrosis and telangiectasia in liver (Fig. 2D) were also detected. Furthermore, hemorrhage and atrophy of seminiferous tubules with necrosis of spermatogenic germ cells were noted in the testis (Figs. 2E and F). Oligospermia and hemorrhage in the epididymis, and atrophy of the BM, thymus, and spleen were observed.
The first successful allogeneic BMT in human was performed in the late 1960s [14]. Science then, BMT has been used with increasing frequency for the treatment of malignant and nonmalignant hematologic diseases, solid tumors, and metabolic and genetic diseases [5]. However, precise regulation of therapeutic gene expression in vivo is still a challenge and the specificity and safety gene therapy needs to be improved even further [13]. Autoimmune-like complication represents a major transplant-related complication initiated by host-reactive T-cells after BMT, and the systemic effects of this initial donor anti-host reaction comprise multi-organ syndrome [6].
CNS hemorrhage is the most prevalent autopsy finding in patient mortality after BMT [2]. In the case reported, an induction of an autoimmune-like complications following BMT was accompanied by severe heart failure with massive myocardial hemorrhage and infiltration by donor-derived CD8-positive cells [12]. Autoimmune-like complications following BMT is also associated with pulmonary edema and cyst formation [14], gonadotoxicity [16], and morphological and functional alterations of thymus [8]. Moreover, autoimmune-like complications remain one of the persistent problems of allogeneic BMT, mostly affecting the skin, gut and the liver [12].
In our case, a large number of infiltrating RBCs were seen in the cerebellar parenchymal spaces, heart, systemic subcutaneous, gastrointestinal tract and liver. Hemorrhage and atrophy of testis and thymus were also observed, and these findings correspond with the previous results mentioned above. There are several factors mentioned in the literature that can be associated with systemic hemorrhage of these multiple organs after BMT. They include a low platelet count or platelet refractoriness underlying BMT [3,15]. And humans with low WBC counts after allogeneic BMT had a significantly higher risk of primary failure of engraftment, or death due to infection, hemorrhage, or graft failure compared to those with higher leukocyte counts [11]. Moreover, it was reported that the BM cytology for allogeneic BM transplanted mice showed displastic BM features in erythroid, myeloid and megakaryocytic cell lineages [15]. In our case, the low platelet and WBC counts in peripheral blood as well as severe depletion of hematopoietic cell in BM were noted. Further studies on cytokine profiles and subsets of leukocytes in blood for specifying the type of autoimmunity may give us more information.
Figures and Tables
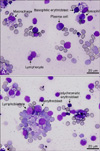 | Fig. 1Bone marrow (BM) cytology from the mouse found moribund 19 days after BM transplantation (BMT). BM cytology showed a severe loss of hematopoietic cells. (A) Few basophilic erythroblasts, basophils, macrophages, plasma cells, and lymphoblasts. (B) Proerythroblasts, polychromatic erythroblasts, and lymphocytes are present. Wright-Geimsa stain. |
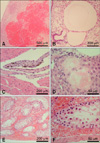 | Fig. 2Histopathological changes of the mouse found moribund 19 days after BMT. (A) Infiltration of large number of red blood cells is seen in the cerebellar parenchyma. (B) Cysts are seen in the pulmonary parenchyma. (C) Hemosiderin deposition is seen in heart myocytes. (D) Hepatocellular necrosis and dilated sinusoid are seen in the liver. (E, F) Hemorrhage in the testicular interstitial space, atrophy of seminiferous tubules, and necrosis of spermatogenic germ cells are noted. H&E stain. |
References
2. Bleggi-Torres LF, de Medeiros BC, Werner B, Neto JZ, Loddo G, Pasquini R, de Medeiros CR. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000. 25:301–307.

3. Bleggi-Torres LF, Werner B, Gasparetto EL, de Medeiros BC, Pasquini R, de Medeiros CR. Intracranial hemorrhage following bone marrow transplantation: an autopsy study of 58 patients. Bone Marrow Transplant. 2002. 29:29–32.

4. Gilman AL, Kooy NW, Atkins DL, Ballas Z, Rumelhart S, Holida M, Lee N, Goldman F. Complete heart block in association with graft-versus-host disease. Bone Marrow Transplant. 1998. 21:85–88.

5. Graus F, Saiz A, Sierra J, Arbaiza D, Rovira M, Carreras E, Tolosa E, Rozman C. Neurologic complications of autologous and allogeneic bone marrow transplantation in patients with leukemia: a comparative study. Neurology. 1996. 46:1004–1009.

6. Hakim F, Fowler DH, Shearer GM, Gress RE. Animal models of acute and chronic graft-versus-host disease. Curr Protoc Immunol. 2001. Chapter 4, Unit 4.3.

7. Haviernik P, Zhang Y, Bunting KD. Retroviral transduction of murine hematopoietic stem cells. Methods Mol Biol. 2008. 430:229–241.

8. Lapp WS, Ghayur T, Mendes M, Seddik M, Seemayer TA. The functional and histological basis for graft-versus-host-induced immunosuppression. Immunol Rev. 1985. 88:107–133.

9. Levy S, Nagler A, Okon S, Marmary Y. Parotid salivary gland dysfunction in chronic graft-versus-host disease (cGVHD): a longitudinal study in a mouse model. Bone Marrow Transplant. 2000. 25:1073–1078.

10. Liao P, Toro A, Min W, Lee S, Roifman CM, Grunebaum E. Lentivirus gene therapy for purine nucleoside phosphorylase deficiency. J Gene Med. 2008. 10:1282–1293.

11. Mehta J, Powles R, Singhal S, Horton C, Middleton G, Eisen T, Meller S, Pinkerton CR, Treleaven J. Early identification of patients at risk of death due to infections, hemorrhage, or graft failure after allogeneic bone marrow transplantation on the basis of the leukocyte counts. Bone Marrow Transplant. 1997. 19:349–355.

12. Platzbecker U, Klingel K, Thiede C, Freiberg-Richter J, Schuh D, Ehninger G, Kandolf R, Bornh-user M. Acute heart failure after allogeneic blood stem cell transplantation due to massive myocardial infiltration by cytotoxic T cells of donor origin. Bone Marrow Transplant. 2001. 27:107–109.

13. Räty JK, Lesch HP, Wirth T, Ylä-Herttuala S. Improving safety of gene therapy. Curr Drug Saf. 2008. 3:46–53.

14. Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest. 1996. 109:1066–1077.

15. Sprangers B, Van Wijmeersch B, Luyckx A, Sagaert X, De Somer L, Rutgeerts O, Lenaerts C, Landuyt W, Boeckx N, Dubois B, De Wolf-Peeters C, Waer M, Billiau AD. Allogeneic bone marrow transplantation and donor lymphocyte infusion in a mouse model of irradiation-induced myelodysplastic/myeloproliferation syndrome (MD/MPS): evidence for a graft-versus-MD/MPS effect. Leukemia. 2009. 23:340–349.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download