1. Azawi OI. Postpartum uterine infection in cattle. Anim Reprod Sci. 2008. 105:187–208.

2. Bagnato A, Oltenacu PA. Phenotypic evaluation of fertility traits and their association with milk production of Italian Friesian cattle. J Dairy Sci. 1994. 77:874–882.

3. Bell MJ, Roberts DJ. The impact of uterine infection on a dairy cow's performance. Theriogenology. 2007. 68:1074–1079.

4. Berry DP, Buckley F, Dillon P, Evans RD, Rath M, Veerkamp RF. Genetic relationships among body condition score, body weight, milk yield, and fertility in dairy cows. J Dairy Sci. 2003. 86:2193–2204.

5. Bonnett BN, Etherington WG, Martin SW, Johnson WH. The effect of prostaglandin administration to Holstein-Friesian cows at Day 26 postpartum on clinical findings, and histological and bacteriological results of endometrial biopsies at Day 40. Theriogenology. 1990. 33:877–890.

6. Bonnett BN, Martin SW, Gannon VP, Miller RB, Etherington WG. Endometrial biopsy in Holstein-Friesian dairy cows. III. Bacteriological analysis and correlations with histological findings. Can J Vet Res. 1991. 55:168–173.
7. Bonnett BN, Martin SW, Meek AH. Associations of clinical findings, bacteriological and histological results of endometrial biopsy with reproductive performance of postpartum dairy cows. Prev Vet Med. 1993. 15:205–220.

8. Bosu WT, Peter AT. Evidence for a role of intrauterine infections in the pathogenesis of cystic ovaries in postpartum dairy cows. Theriogenology. 1987. 28:725–736.

9. Dohmen MJW, Lohuis JACM, Huszenicza G, Nagy P, Gacs M. The relationship between bacteriological and clinical findings in cows with subacute chronic endometritis. Theriogenology. 1995. 43:1379–1388.

10. Dolezel R, Vecera M, Palenik T, Cech S, Vyskocil M. Systematic clinical examination of early postpartum cows and treatment of puerperal metritis did not have any beneficial effect on subsequent reproductive performance. Vet Med. 2008. 53:59–69.

11. Edmonson AJ, Lean IJ, Weaver LD, Farver T, Webster G. A body condition scoring chart for Holstein dairy cows. J Dairy Sci. 1989. 72:68–78.

12. González-Recio O, Alenda R, Chang YM, Weigel KA, Gianola D. Selection for female fertility using censored fertility traits and investigation of the relationship with milk production. J Dairy Sci. 2006. 89:4438–4444.

13. Huzzey JM, Veira DM, Weary DM, von Keyserlingk MAG. Prepartum behavior and dry matter intake identify dairy cows at risk for metritis. J Dairy Sci. 2007. 90:3220–3233.

14. Kim IH. Risk factors for delayed conception in Korean dairy herds. J Vet Sci. 2006. 7:381–385.

15. Königsson K, Gustafsson H, Gunnarsson A, Kindahl H. Clinical and bacteriological aspects on the use of oxytetracycline and flunixin in primiparous cows with induced retained placenta and post-partal endometritis. Reprod Domest Anim. 2001. 36:247–256.

16. LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci. 2002. 85:2223–2236.

17. LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. The effect of treatment of clinical endometritis on reproductive performance in dairy cows. J Dairy Sci. 2002. 85:2237–2249.

18. LeBlanc SJ, Lissemore KD, Kelton DF, Duffield TF, Leslie KE. Major advances in disease prevention in dairy cattle. J Dairy Sci. 2006. 89:1267–1279.

19. Lee JY, Kim IH. Advancing parity is associated with high milk production at the cost of body condition and increased periparturient disorders in dairy herds. J Vet Sci. 2006. 7:161–166.

20. Lewis GS. Uterine health and disorders. J Dairy Sci. 1997. 80:984–994.

21. Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. 1999. Cary: SAS Institute;423–460.
22. López-Gatius F, Santolaria P, Yániz J, Fenech M, López-Béjar M. Risk factors for postpartum ovarian cysts and their spontaneous recovery or persistence in lactating dairy cows. Theriogenology. 2002. 58:1623–1632.

23. Mateus L, da Costa LL, Bernardo F, Silva JR. Influence of puerperal uterine infection on uterine involution and postpartum ovarian activity in dairy cows. Reprod Domest Anim. 2002. 37:31–35.

24. Miller AN, Williams EJ, Sibley K, Herath S, Lane EA, Fishwick J, Nash DM, Rycroft AN, Dobson H, Bryant CE, Sheldon IM. The effects of
Arcanobacterium pyogenes on endometrial function
in vitro, and on uterine and ovarian function
in vivo. Theriogenology. 2007. 68:972–980.

25. Opsomer G, Gröhn YT, Hertl J, Coryn M, Deluyker H, de Kruif A. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: a field study. Theriogenology. 2000. 53:841–857.

26. Peter AI, Bosu WI. Influence of intrauterine infections and follicular development on the response to GnRH administration in postpartum dairy cows. Theriogenology. 1988. 29:1163–1175.

27. Runciman DJ, Anderson GA, Malmo J, Davis GM. Use of postpartum vaginoscopic (visual vaginal) examination of dairy cows for the diagnosis of endometritis and the association of endrometritis with reduced reproductive performance. Aust Vet J. 2008. 86:205–213.

28. Smith MCA, Wallace JM. Influence of early post partum ovulation on the re-establishment of pregnancy in multiparous and primiparous dairy cattle. Reprod Fertil Dev. 1998. 10:207–216.

29. Tsousis G, Sharifi A, Hoedemaker M. Increased risk of conception failure in German Holstein Friesian cows with chronic endometritis. Reprod Domest Anim. 2009. Epub ahead of print. doi:
10.1111/j.1439-0531.2009.01481.x.

30. Veerkamp RF, Koenen EPC, De Jong G. Genetic correlations among body condition score, yield, and fertility in first-parity cows estimated by random regression models. J Dairy Sci. 2001. 84:2327–2335.

31. Williams EJ, Fischer DP, Noakes DE, England GC, Rycroft A, Dobson H, Sheldon IM. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology. 2007. 68:549–559.

32. Williams EJ, Fischer DP, Pfeiffer DU, England GC, Noakes DE, Dobson H, Sheldon IM. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology. 2005. 63:102–117.

33. Wood PDP. Algebraic model of the lactation curve in cattle. Nature. 1967. 216:164–165.

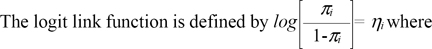 where πi is the probability of occurrence recorded from day 21 p.p. and until the date of successful a.i. or culling.
where πi is the probability of occurrence recorded from day 21 p.p. and until the date of successful a.i. or culling.



 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download