Abstract
In this study, we evaluated if the implantation of allogenic adipose-derived stem cells (ASCs) improved neurological function in a canine spinal cord injury model. Eleven adult dogs were assigned to three groups according to treatment after spinal cord injury by epidural balloon compression: C group (no ASCs treatment as control), V group (vehicle treatment with PBS), and ASC group (ASCs treatment). ASCs or vehicle were injected directly into the injured site 1 week after spinal cord injury. Pelvic limb function after transplantation was evaluated by Olby score. Magnetic resonance imaging, somatosensory evoked potential (SEP), histopathologic and immunohistichemical examinations were also performed. Olby scores in the ASC group increased from 2 weeks after transplantation and were significantly higher than C and V groups until 8 weeks (p < 0.05). However, there were no significant differences between the C and V groups. Nerve conduction velocity based on SEP was significantly improved in the ASC group compared to C and V groups (p < 0.05). Positive areas for Luxol fast blue staining were located at the injured site in the ASC group. Also, GFAP, Tuj-1 and NF160 were observed immunohistochemically in cells derived from implanted ASCs. These results suggested that improvement in neurological function by the transplantation of ASCs in dogs with spinal cord injury may be partially due to the neural differentiation of implanted stem cells.
Central nervous system regeneration is highly limited after injury. Spinal cord injury (SCI) leads to cell death, particularly in neurons, oligodendrocytes, astrocytes, and precursor cells [12]. Any cavities and cysts resulting from this cell death and loss may interrupt axonal tracts. SCI culminates in glial scarring, a multifactorial process involving reactive astrocytes, glial progenitors, microglia, macrophages, fibroblasts, and Schwann cells [15]. Such scars are often oriented perpendicular to the neuraxis, contain transmembrane molecular inhibitors of axon growth, and appear impenetrable [34]. Neuronal phenotypes are not generated following SCI [42] and apparent lack of regenerative capacities of the adult spinal cords could result from the neurogenesis inhibitors of myelin-derived proteins, glial scar and extracellular matrix-derived factor [35].
Cell transplantation therapy using adult stem cells has recently been identified as a potential treatment for SCI [2]. Such cells can differentiate into appropriate neuronal phenotypes in ischemic or damaged brain and spinal cord [18]. Adipose tissue compartments are a particularly useful source of mesenchymal stem cells (MSCs) due to ease of harvest, clonogenic potential, and robust proliferative capacity [7]. Adipose-derived stem cells (ASCs) can differentiate into adipocyte, chondrocyte, myocyte, osteoblast, and even neural lineages [11].
ASCs may have therapeutic potential for neurological disorders, and functional recovery after transplantation of ASCs into the areas of spinal cord injury in vivo was reported in rodent models of SCI [18]. Rodent spinal cords are smaller than canine cords and are also anatomically distinct in areas such as the extrapyramidal tract, therefore the rodent model is not suitable for detailed physical analyses or accurate evaluation of recovery [10]. Although it was reported that umbilical cord blood derived MSCs was effective in canine SCI model, there was little histological evidence of spinal cord tissue regeneration [22]. In this study, we examined whether canine ASCs could survive and integrate into neural cells and the effectiveness of canine ASCs on the improvement of neurological function in canine SCI model.
Eleven healthy adult mixed-breed dogs (4.6 ± 0.4 kg) were used. Applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research. This investigation was performed in accordance with the guidelines of the "Guide for the Care and Use of Laboratory Animals" of Seoul National University. SCI was induced by epidural ballon compression. The dogs were randomly assigned to 3 groups based on post-SCI treatment (31): Group C, control group with no ASCs transplantation (n = 3); Group V, vehicle group with phosphate-buffered saline (PBS) injection (n = 3); Group ASC, group with transplantation of allogenic ASCs into the site of SCI (n = 5).
Adipose tissue was aseptically collected from the subcutaneous fat of a 2-year-old experimental dog under anesthesia. Tissues were washed extensively with PBS, minced and digested with collagenase type I (1 mg/mL; Sigma, USA) at 37℃ for 2 h [16]. After washing with PBS and centrifuging at 4℃, pellets of stromal vascular fraction (SVF) were resuspended, filtered through 100 µm nylon mesh and incubated overnight in DMEM with 10% fetal bovine serum (FBS; Gibco BRL, USA) at 37℃ with 5% humidified CO2. Unattached cells and residual non-adherent red blood cells were removed after 24 h by washing with PBS, and cell medium was exchanged with Keratinocyte-SFM (Gibco BRL, USA). The medium was supplemented with human recombinant epidermal growth factor (rEGF, 5 ng/mL; Gibco BRL, USA), bovine pituitary extract (50 µg/mL; Gibco BRL, USA), 2 mM N-acetyl-L-cysteine (NAC; Sigma, USA), 0.2 mM L- ascorbic acid 2-phosphate (Asc 2P; Sigma, USA), insulin (5 µg/mL; Sigma-Aldrich, USA), hydrocortisone (74 ng/mL; Sigma-Aldrich, USA). Medium was changed at 48 h intervals until the cells became confluent. After cells reached 90% confluence, they were trypsinized and stored in liquid nitrogen or subcultured at a density of 10,000 cells/cm2 (passage 1). Cells were passage repeatedly after achieving a density of 80~90% (approximately 7 days in culture) until passage 8.
ASCs were differentiated in culture under the conditions described below.
Adipogenic differentiation: ASCs were initially cultured and propagated up to 80~90% confluence in K-NAC medium containing 5% FBS and then shifted to adipogenic medium [DMEM high-glucose medium with 10% FBS, 10 µg/mL insulin (Sigma-Aldrich, USA), 1 µM dexamethasone (Sigma-Aldrich, USA), 0.2 mM indomethacin (Sigma-Aldrich, USA), and 0.5 mM isobutylmethylxanthine (Sigma-Aldrich, USA)] for 3 days, then to DMEM high-glucose medium with 10% FBS, 10 µg/mL insulin (Sigma-Aldrich, USA) for 4 days. This procedures repeated 3 times for 21 days [13]. The accumulation of neutral lipids was detected by staining ASCs in a solution of 0.5% Oil red O.
Osteogenic differentiation: ASCs were initially cultured and propagated up to 70% confluence in K-NAC medium containing 5% FBS and then shifted to osteogenic medium [DMEM low-glucose medium with 10% FBS, 0.1 µM dexamethasone (Sigma-Aldrich, USA), 50 µM l-Ascorbate-2-phosphate (Sigma-Aldrich, USA), and 10 mM beta-glycerophosphate (Sigma-Aldrich, USA)] for 3 weeks [14]. Mineralization was assessed by staining ASCs with 40 mM Alizarin red S (pH 4.1).
Neurogenic differentiation: Neurogenic differentiation was induced by culturing ASCs in preinduction medium [DMEM low-glucose medium supplemented with 10% FBS and 1 mM b-mercaptoethanol (Sigma-Aldrich, USA)] for 24 h. After preinduction, the cells were induced for up to 5 h in neurogenic medium [DMEM with 100 µM butylated hydroxyanisol (Sigma-Aldrich, USA) and 1% DMSO (Sigma-Aldrich, USA)] [39]. The cells were analyzed by immunofluorescence staining for the expression of MAP2 (neuronal lineage) and Oct4 (pluripotent stem cell marker) [16]. ASCs were grown on four-well Lab-Tek slides (Nalge Nunc, USA). After blocking for 2 h in PBS containing 10% normal goat serum (Zymed Laboratories, USA), slides were incubated for 5 h at 4℃ with anti-MAP2 rabbit polyclonal (Chemicon International, USA) and anti-Oct4 rabbit polyclonal (Santa Cruz Biotechnology, USA) antibodies diluted in PBS. Slides were then washed 3 times in PBS and incubated in TRITC goat anti-rabbit secondary antibody (BD Biosciences, USA) for 1 h at room temperature. Slides were washed 3 times in PBS and mounted. For the negative control, primary antibodies were omitted.
ASCs were examined for surface markers using Flow Cytometry [16]. The following antigens were purchased from VMRD (USA) unless otherwise indicated. The first passage of ASCs were analyzed for canine major histocompatibility complex (MHC)-class I (#H58A), MHC-class II (#CAT82A), histocompatibility locus antigen (HLA)-DR (#TH14B), pan-lymphocyte (#DH52A), B lymphocyte (#F46A), neutrophil (#CADO48A), CD4 (#DH29A), CD8 (#CADO46A), CD44 (#BAG40Am), CD45-like (#CADO18A), CD90 (#DH24A), CD14 (#CAM36A), CD3 (#MCA1774; AbD Serotec, USA), CD11c (#MCA1778S; AbD Serotec, USA) and CD34 (#1H6; Becton, Dickinson and Company, USA). The seventh passage of ASCs were trypsinized, centrifuged and resuspended to concentration of about 5 × 105 cells for each test. Thus, 30 µL each of a prediluted PE-conjugated mouse anti-dog CD14 (#CAM46A), a PE-conjugated mouse anti-dog CD34 (MCA2411F; AbD Serotec, USA), a PE-conjugated mouse anti-dog CD45-like (CADO18A), a PE-conjugated rat anti-dog CD44 (ab19622; Abcam, UK), a PE-conjugated mouse anti-dog CD90 (DH2A) and a FITC-conjugated mouse anti-human CD105 (555690; BD Biosciences, USA) antibody was used in individual test. Negative control staining was performed using a FITC-conjugated mouse IgG1 isotype and a PE-conjugated mouse IgG1 isotype antibody respective the primary antibodies.
Some cells were infected with a lentivirus-vector labeled GFP gene. Lentivirus was generated with ViraPower Lentiviral packaging Mix (Invitrogen, USA). Lipofectamine 2000 (Invitrogen, USA) was used for transfection of SHC003 MISSION TurboGFP control vector (Sigma, USA) to 293FT cells (Invitrogen, USA). Cell culture media was changed the day after transfection and supernatant was harvested at 48 and 72 h after transfection. Viral supernatant was filtered using 0.4 µm pore filter (Invitrogen, USA). ASCs were transfected with TurboGFP-lentivirus about 15 multiplicity of infection (MOI). Polybrene (Sigma, USA) was added to cell culture media at a final concentration of 6 µg/mL. Cell culture media was changed the day after transfection with fresh culture media and green fluorescence was identified in cytoplasm of cells 48 h after transfection with a fluorescent microscope (Fig. 2B).
Spinal cords of the experimental dogs under general anesthesia were compressed by epidural ballon catheter for 12 h and resulted in SCI [22]. A fentanyl patch (Durogesic D-trans patch 25 mcg/h 4.2 mg/10.5 cm2; Alza Ireland, Ireland) was used for analgesia 24 h before the operation. Cefazolin sodium (20 mg/kg; Chong Kun Dang Pharm, Korea) was given intravenously (IV) as a prophylactic antibiotic. Atropine sulfate (0.03 mg/kg; Je Il Pharm, Korea) was administered. The dogs were sedated with the IV administration of diazepam (Dong Wha Pharm, Korea) at a dose of 0.2 mg/kg, immediately followed by intravenous morphine (Ha Na Pharm, Korea) at 0.3 mg/kg. The dogs were induced with the IV administration of propofol (Ha Na Pharm, Korea) at 6 mg/kg. Anesthesia was maintained by 2% isoflurane (Ilisung, Korea) in oxygen. The minimum alveolar concentration was about 1.5. A multiparameter anesthetic monitor (Datex-Ohmeda, Denmark) was used to monitor physiologic measures, including rectal temperature, oxygen saturation, end tidal CO2, electrocardiogram, anesthetic agent concentration and blood pressure.
Following anesthetic stabilization, a mini-hemilaminectomy procedure was performed using a median approach to L4. A 3 to 5 mm hole was created in the left vertebral lamina at L4 using a high-speed pneumatic burr. A 3-French embolectomy catheter (Sorin Biomedica, Italy) was inserted into the hole at L4. A balloon was advanced, under fluoroscopic guidance, until the tip of the catheter reach the cranial margin of the L1 vertebral body. The balloon was inflated with a 50:50 solution of contrast agent (Omnipaque; Amersham Health, Ireland) and saline at a dose of 40 µL/kg body weight for 12 h. It took approximately 30 min to induce SCI. The balloon catheter was fixed with a Chinese finger type suture and removed after 12 h. All dogs were administrated analgesics by continuous rate infusion for 18 h after skin closure. Post-operative analgesics contained morphine (Ha Na Pharm, Korea) at 0.15 mg/kg/h, lidocaine HCl (Dai Han Pharm, Korea) at 2 mg/kg/h and ketamine HCl (Yuhan Pharm, Korea) at 0.3 mg/kg/h [26]. After the operation, dogs were bandaged, monitored in the intensive care unit and the degree of pain assessed at 30 min intervals. The dogs with some overt signs of discomfort were given IV morphine at 0.2 mg/kg additionally.
Suture materials were removed after 10 days. Dogs were fed with a nutritionally balanced feed twice a day and if necessary, manual bladder expression was performed at least three times daily until voluntary urination was established. The general condition of the dogs and their neurological status was monitored twice daily during the time of the study and there were no complications except for mild cystitis and muscle atrophy of hind limbs. Two dogs had a seroma in the surgical site and recovered spontaneously after 2 weeks.
ASCs were transplanted 1 week after experimentally-induced SCI. Group C did not receive media or any transplanted cells. For group V, the injured site was exposed by dorsal laminectomy and 150 µL of PBS was injected into the spinal cord at 3 locations to depths of 3 mm using a 30 gauge needle (middle of the injury site, proximal and distal margins). For group ASC, 1 × 106 of prepared cells suspended in 150 µL PBS were injected into the SCI site in same fashion as group V. One dog in the ASC group was injected with GFP-labeled ASCs.
Using a 15-point scoring system (Olby score), the dogs' gaits were independently scored from videotapes by 2 separate individuals who were blinded to the experimental conditions [37]. Mean scores at 1, 3, 5 and 9 weeks after SCI were calculated.
Somatosensory evoked potentials (SEP) were measured using a Neuropack 2 (Nihon Kohden, Japan) and two subdermal channels at 1, 5, and 9 weeks after the cell transplantation. Channel 1 was installed at the subdermal region at the midline between the sixth and seventh lumbar vertebrae (L6-L7) and channel 2 was installed between the tenth and eleventh thoracic vertebrae (T10-T11) using platinum grass stimulating electrode needle (Astro-Med, USA). The posterior tibial nerve was stimulated for 0.2 msec, with 2 Hz and 3 mA [41]. The latency response was converted into velocity as a measure of spinal cord dysfunction. The spinal conduction velocity from the 6th lumbar (L) vertebra to the 10th thoracic (T) vertebra was calculated by the following equation: Conduction velocity (m/sec) = [distance between 2 points (cm)/latency difference (msec)] × 10.
Magnetic resonance image (MRI) was performed using a 0.2 Tesla Magnet scanner (Esaote, Italy). A majority of the obtained images were interleaved at 5.0 mm with a slice thickness of 5.0 mm. The repetition time (TR) and time to echo (TE) were adjusted. T1-weighted (TR/TE = 540/26 msec, T1W) and T2-weighted (TR/TE = 380/90 msec, T2W) echo images were obtained. All dogs in each group were examined and the SCI lesions were expressed in T2W sagittal planes at 5 and 9 weeks after the injury.
All dogs were euthanized 9 weeks after spinal cord injury. The dogs were sedated with IV administration of diazepam (Dong Wha Pharm, Korea) at a dose of 0.2 mg/kg immediately followed by IV morphine at 0.3 mg/kg. The dogs were induced with IV administration of propofol (Ha Na Pharm, Korea) at 6 mg/kg. After tracheal intubation, anesthesia was maintained by isoflurane (Ilisung, Korea) in oxygen. The dogs were euthanized by pentobarbital sodium (Han Lim Pharm, Korea) at 80 mg/kg and bolus injection of 10 mL KCl solution (1 M) into the cephalic vein. The spinal cords from T10 to L4 of all dogs were sampled. Spinal cords were fixed in 20% sucrose solution overnight at 4℃. Dura were removed by scissors, embedded using O.C.T compound (Sakura Finetechnical, Japan), frozen and transversely sectioned at epicenter of lesion. These sections were mounted on silanecoated glass slides.
Slides were stained first with hematoxylin and eosin, and then with combined Luxol fast blue and cresyl violet to identify myelin and nerve cells [10]. Percentages of myelinated areas in damaged spinal cords were calculated using the formula, (myelinated areas/total area) × 100, from images of the transverse sections using image analyzer software (ImageJ version 1.37; National Institutes of Health, USA). Longitudinal sections were made with tissue in which GFP-labeled lentiviral vector inserted stem cells were injected. Primary antibodies were used against mature astrocytes (GFAP, AB5804; Chemicon International, USA), immature neurons (TUJ1-β, ab14545; Abcam, UK), motor neurons (NF160, N5264; Sigma, USA), and oligodendrocytes (Oligodendrocyte marker, MAB5540; Chemicon International, USA) for immunofluorescent determinations of the phenotypes of GFP (+) cells. Tissues were incubated in goat serum for 2 h at room temperature. The tissues were then incubated with the primary antibodies for 24 h at 4℃. Secondary antibodies (anti-mouse fluro 588, anti-rabbit fluro 588; Invitrogen, USA) were used against primary antibodies. DAPI (1 : 100; Sigma, USA) was added to a final wash to identify nuclei. Tissues were mounted with aqueous mounting medium (Dakocytomation, USA) and kept in the dark at -4℃ until analysis. Slide images were obtained by confocal microscopy (Nikon, Japan).
Results were expressed as medians for Olby scores and the means ± SD for SEP values and Luxol fast blue positive areas. Statistical analysis used SPSS 12.0 software (SPSS, USA). Kruskal-Wallis analysis for Olby scores and oneway ANOVA for SEP values and Luxol fast blue positive areas were used. p-value < 0.05 was considered significant.
Adipogenic differentiation of ASCs was apparent after 3 weeks of incubation with adipogenic medium. By the end of the third week, half of the cells contained Oil red O-positive lipid droplets (Fig. 1B). The colonies of ASCs were subjected to Alizarin red S staining 3 weeks after the initiation of osteogenic differentiation. Intense Alizarin red S staining of the colonies confirmed that calcium deposition had occurred (Fig. 1D). After ASCs were induced into neurogenic differentiation, cells stained positive for the neuronal marker MAP2 and were negative for the undifferentiated marker Oct-4 during neuronal differentiation in vitro.
The first passage of ASCs expressed CD44, CD90, CD105 and MHC class I, and were also partially positive for CD34. They did not express CD14 or CD45. The seventh passage of ASCs expressed CD44, CD90 and CD105, and were negative for CD14, CD34, CD45 (Fig. 3).
Olby scores for all groups were 0 points post-SCI, at the start of treatment. The scores for the ASC group increased to 1, 3.6 and 4.6 points at 3, 5 and 9 weeks, respectively (Fig. 4). Scores in the C and V groups remained below 1 point up to the end of the study. Scores for the ASC group were significantly higher than those for the C and V groups at 5 weeks (p < 0.05). There were no significant differences between the C and V groups.
It was possible to measure evoked potentials in the ASC group 5 weeks post-injury. The C and V groups had no responses at to 9 weeks. Mean conduction velocities in the ASC group were 22.8 ± 10.9 m/sec at 5 weeks and 31.1 ± 12.2 m/sec at 9 weeks.
MRI scans were well tolerated by all dogs. The majority of dogs in all groups showed clear, hyper-intense signals in the T2W sagittal plane of the lesion at the 1st lumbar vertebra (L1) at 1 week and 5 weeks after SCI. T2W images showed reduction of swelling and hyperintense signal at 9 weeks in all groups. These hyper-intense signals were not different among groups (Fig. 5).
Margins for gray and white matters were not identified in any of the dogs at 9 weeks (Figs. 5 and 7). There were generalized infiltrations of fibrous tissues and adhesions in the dura mater. Most dogs had mild vacuolar formations. Cavitation of the gray matter was seen within cranial and caudal lesions of the SCI site. The areas positively stained Luxol fast blue in the ASC group were larger than those in other groups. The mean percentages of Luxol fast blue positive areas in the C, V and ASC groups were 16.66 ± 2.41%, 17.06 ± 2.85% and 31.16 ± 3.13%, respectively (p < 0.05) (Fig. 6). High magnification revealed neuronal cell like structures. GFP positive cells were stained by Tuj-1 in serial transverse sections (Fig. 7). In longitudinal sections of the lesion, GFP positive cells were observed and were also positive for GFAP, NF160, Tuj-1 and oligodendrocytes (Fig. 8).
The SVF contains an unpurified population of stromal cells, which includes ASCs. The other cell types that may be present in SVF are endothelial cells, smooth muscle cells, pericytes, fibroblasts, and circulating cell types such as leucocytes, hematopoietic stem cells or endothelial progenitor cells [44]. Many studies have used the entire unpurified SVF in their experiments on the basis that the ASC are adherent to the plastic tissue cultureware, so they are self-selected out of the SVF during subsequent tissue culture passages [27]. As few as one in 30 of the SVF cells adhere to the plastic [25], and there is a progressive loss of hematopoietic lineage cell markers (such as CD11, CD14 and CD45) with successive cultures of ASC [25]. Adherence to plastic tissue cultureware, however, is not a feature that is specific to ASCs because fibroblast cells also behave in this manner. Some critics have suggested that even a low fraction of contaminating cells such as hematopoietic stem cells could be the source of differentiation seen in ASC experiments [32]. Purification by magnetic bead coupling has been performed [4] to remove CD45+ cells (leucocytic/ hematopoietic lineage) and CD31+ cells (endothelial lineage) from the isolated cells prior to differentiation experiments. Given the relative simplicity of such sorting procedures, it would seem reasonable to advocate that ASCs should be purified from the SVF before cell culture. In our study, adipose tissue culture yielded an adherent growing cell population with a spindle morphology. Flow cytometric analysis revealed high levels of CD44, CD90 and CD105 expression whereas the expression of CDproteins typical for hematopoietic cells remained undetectable. It identified ASCs as partially positive for CD34 in SVF preparation, but this marker is subsequently lost during in vitro culture [4]. These findings suggested the presence of mesenchymal stem cell-like cells according to the standard criteria for MSCs from the International Society for Cellular Therapy [8]. Moreover, a distinct subpopulation of the ASCs demonstrated the potential to differentiate into adipocyte, osteocyte, and neuron-like cells.
SCI has been investigated by using various experimental models such as weight drop [23], pneumatic impaction [1], and extradural balloon compression [19]. The major factor in the pathogenesis of SCI produced by the weight dropping method was mechanical, whereas both mechanical and vascular factors were involved in balloon compression methods. Balloon compresses the spinal cord and produces a closed injury without laminectomy at the injury site, and thus it resembles injuries observed in clinical cases of, for example, unreduced dislocation, intervertebral disc disease or fracture dislocation [10]. The balloon-induced method has been used because it is a simple method that does not cause any damage to the surrounding structures and dose-response based on volume of the balloon and degree of injury occurs in rats and dogs [36]. Our method of drilling a mini hemi-laminectomy hole for insertion of a balloon catheter provided easy exposure of dura mater with no risk of hemorrhage in a relatively short time (30 min).
In this study, the SCI model resulted in over 75% spinal canal occlusion by balloon compression over 12 h. Severe hemorrhage and vacuolar formation occurred 1 week after SCI and generalized infiltration of fibrous tissue was seen 9 weeks post-injury and no functional improvement in control group were observed. Similar histopathological findings at 9 weeks have been previously reported [22]. Margins for gray and white matter were not identified in any of the dogs at 9 weeks post-injury. There were generalized infiltrations of fibrous tissues and adhesions in the dura mater. Most dogs had mild vacuolar formations. Cavitation of the gray matter was seen within cranial and caudal lesions of the SCI site. Vacuolar formations and cavitation acted as a physical barrier to the growth of anatomically intact axons. There were no myelinated axons and normal neurons in epicenter of SCI lesions in all dogs.
Classically, the Tarlov scale has been used for the quantitative evaluation of neurological status resulting from spinal cord injury in dogs [31]. Basso-Bresnahan-Beattie (BBB) score for rodents or modification of the Tarlov scale also have been used [20], but those scoring systems were not sensitive enough to describe the details of functional status due to the large variations resulting from the broad category of each level. Olby et al. [28] modified the BBB open field scoring system for dogs based on the pelvic limb gait of dogs with SCI resulting in thoracolumbar vertebral disc herniations. The pelvic limb gaits of dogs who recover from SCI can be reliably quantified with a numeric scale, namely, the Olby score [28]. In our study, dogs had Olby scores < 1 up to 9 weeks after injury in the C and V groups. An Olby score of 1 is defined as a neurological status for which there is no pelvic limb movement and with deep pain sensation. The Olby score for the ASC group increased in the 9 weeks after injury and was 4.6 at the end of the study, with moveable joints of the pelvic limbs. This score at 9 weeks was lower than that a previous report (Olby score; 7.4) that used umbilical cord blood-derived stem cells [22].
Conduction velocities calculated from SEP amplitudes and latencies have been associated with the severity of spinal cord damage in experimentally induced SCI [29]. SEP conduction velocities in dogs with mild spinal cord lesions were lower than those in normal dogs [29]. The SEP has a flat waveform when the spinal cord is injured by more than 50% [21]. The neurological status of the C and V groups was consistent with a flat waveform up to 9 weeks post-injury. The mean conduction velocity in the ASC group at 9 weeks was 31.1 ± 12.2 m/sec, which is approximately 50% lower than that in normal dogs [41].
MRI is a useful and powerful tool in detection and characterization of spinal cord pathology in animal models [3,38]. T1 weighted images were considered most useful for assessment of cord swelling and hemorrhage, and T2 weighted images were valuable for assessment of fluid infiltration into the cord, i.e. edema [24]. Cord swelling occurs due to disruption of vasculature and alteration of local fluid compartmentalization, with subsequent accumulation of blood and edema in and around the site of the contusion injury [9]. The hypointense areas in the L1 parenchyma on T2W images could be considered a black boundary artifact [24]. The hyperintense lesions at 5 and 9 weeks after transplantation were not different among groups. Our MRI settings were useful for the identification of localized spinal cord lesions. However, they were not sufficient to show changes of spinal cord lesions in the chronic phase.
In vivo MSC studies have demonstrated the cellular fate of cells which integrated into injured spinal cord [33]. Recent reports have suggested that ASCs survived and migrated to injured CNS tissue after transplantation [17], and transplanted MSCs express GFAP or neuronal nuclear antigen in the ischemic brain [43]. In this study, GFP-labeled stem cells inserted with lentiviral vectors were positive for GFAP, NF160, Tuj-1, and an oligodendrocyte marker in spinal cord lesions. This suggested that the implanted ASCs differentiated into astrocytes and oligodendrocytes, as well as neuronal cells. Neurons derived from engrafted cells may relay signals from disrupted fibers in the host, including local circuit interneurons or ascending fibers that are present in the dorsal column [5].
The neuronal transdifferentiation processes seen for ASCs may result from the interactions of cells, cytokines provided by these cells, growth factors and intercellular signals [6]. ASCs have been shown to secrete multiple angiogenic and anti-apoptotic cytokines that support tissue regeneration and minimize tissue damage [30]. Engrafted ASCs and SCI-induced chemotactic factors play important roles in proliferation, migration and differentiation of endogenous spinal cord-derived neural progenitor cells in an injured region [18]. Those MSCs that survived produced large amounts of basic fibroblast growth factor and vascular endothelial growth factor receptor 3 in the host spinal cord [40].
In conclusion, these results suggest that improvements of neurological function after transplantation of ASCs to dogs with spinal cord injuries might be partially due to neural differentiation of implanted stem cells.
Figures and Tables
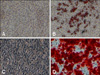 | Fig. 1Adipogenic and osteogenic differentiation of canine adipose-derived stem cells (ASCs). A: ASCs cultured in DMEM + 10% FBS media (control media), not stained by Oil red O. B: Oil red O stained after 3 weeks incubation at adipogenic media. C: ASCs cultured in control media, not stained with Alizarin red S. D: Intense Alizarin red S stained after 3 weeks incubation at osteogenic media and confirmed calcium deposition. A and B: Oil red O stain, C and D: Alizarin red S stain, ×100. |
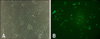 | Fig. 2Green fluorescence protein (GFP) labeled canine adipose-derived stem cells (ASCs). (A) Typical morphological feature of canine ASCs. (B) Green fluorescence was identified in ASCs at 48 h after transfection. ×100. |
 | Fig. 3Flow cytometric analysis of surface-marker expression on ASC. The seventh passage of ASCs expressed CD44, CD90 and CD105, and were negative for CD14, CD34, CD45. The overwhelming majority (> 95%) of cASC expressed the mesenchymal cell surface markers CD90 and CD105. |
 | Fig. 4Olby scores during the 9 week post-SCI study period. The scores in ASC group were significantly higher than these in the other two groups at 5 and 9 weeks after spinal cord injury (*p < 0.05). |
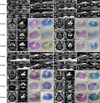 | Fig. 5Images of the spinal cord injury lesion. A: control group with no ASCs transplantation. B: vehicle group with PBS. C and D: ASC group with transplantation with ASCs. Sagittal image: a, b, c and d. T1-weighted MR image at 5 weeks (a), T2-weighted MR image at 5 weeks (b), T1-weighted MR image at 9 weeks (c), T2-weighted MR image at 9 weeks (d). White arrowhead indicated the cranial, center and caudal portions of the transverse image. Transverse, T1-weighted MR image at 5 weeks (T1) and transverse, T2-weighted MR image at 5 weeks (T2). Black arrow indicated cavitation. The hyperintense lesions in T2-weighted MR image at 5 and 9 weeks after spinal cord injury were not different among groups. |
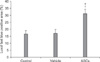 | Fig. 6Percentage of luxol fast blue staining positive areas in the transverse sections at the epicenters of injured spinal cords. Luxol fast blue positive areas in the control group and vehicle group were smaller than those in the ASC group. *p < 0.05 compared to control gruop, †p < 0.05 compared to vehicle group. |
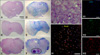 | Fig. 7Histopathological findings 9 weeks after spinal cord injury. A and D: group C; B and E : group V; C, F-H: group ASC. All groups showed extensively damaged tissues. F: Positive areas for Luxol fast blue staining were observed at injured sites in the ASC group (circles). G and H: These showed structural consistency with nerve cell. A, B and C: H&E stain, D-G: Luxol fast blue and cresyl violet stain, H, H1, H2 and H3: Immunofluorescence staining. Scale bars = 50 µm. |
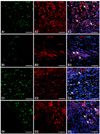 | Fig. 8Immunofluorescence staining of ASC group; A1-A3: Glial fibrillary acidic protein (GFAP), B1-B3: Neurofilament M (NF160), C1-C3: Neuronal class III beta tubulin (Tuj-1), D1-D3: Oligodendrocyte, Green fluorescence protein (GFP)- labeled lentiviral vector inserted stem cells were positive with GFAP, NF 160, Tuj-1, and oligodendrocytes in injured lesions (arrows). Scale bars = 50 µm. |
Acknowledgments
This work was supported by the Research Institute for Veterinary Science, Seoul National University, the BK21 Program for Veterinary Science and the Seoul R&BD Program (10548).
References
1. Anderson TE. A controlled pneumatic technique for experimental spinal cord contusion. J Neurosci Methods. 1982. 6:327–333.

2. Barnett SC, Riddell JS. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair--what can it achieve? Nat Clin Pract Neurol. 2007. 3:152–161.

3. Berens SA, Colvin DC, Yu CG, Yezierski RP, Mareci TH. Evaluation of the pathologic characteristics of excitotoxic spinal cord injury with MR imaging. AJNR Am J Neuroradiol. 2005. 26:1612–1622.
4. Boquest AC, Shahdadfar A, Frønsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005. 16:1131–1141.

5. Bregman BS, Kunkel-Bagden E, Reier PJ, Dai HN, McAtee M, Gao D. Recovery of Function after Spinal Cord Injury: Mechanisms Underlying Transplant-Mediated Recovery of Function Differ after Spinal Cord Injury in Newborn and Adult Rats. Exp Neurol. 1993. 123:3–16.

6. Cova L, Ratti A, Volta M, Fogh I, Cardin V, Corbo M, Silani V. Stem cell therapy for neurodegenerative diseases: the issue of transdifferentiation. Stem Cells Dev. 2004. 13:121–131.

7. Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004. 22:560–567.

8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.

9. Duncan EG, Lemaire C, Armstrong RL, Tator CH, Potts DG, Linden RD. High-resolution magnetic resonance imaging of experimental spinal cord injury in the rat. Neurosurgery. 1992. 31:510–519.

10. Fukuda S, Nakamura T, Kishigami Y, Endo K, Azuma T, Fujikawa T, Tsutsumi S, Shimizu Y. New canine spinal cord injury model free from laminectomy. Brain Res Brain Res Protoc. 2005. 14:171–180.

11. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003. 5:362–369.

12. Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006. 498:525–538.

13. Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004. 6:543–553.

14. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.

15. Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003. 182:399–411.

16. Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008. 17:681–693.

17. Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003. 183:355–366.

18. Kang SK, Shin MJ, Jung JS, Kim YG, Kim CH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006. 15:583–594.

19. Kobrine AI, Evans DE, Rizzoli HV. Experimental acute balloon compression of the spinal cord. Factors affecting disappearance and return of the spinal evoked response. J Neurosurg. 1979. 51:841–845.
20. Kuh SU, Cho YE, Yoon DH, Kim KN, Ha Y. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir (Wien). 2005. 147:985–992.

21. Lee JM. Evaluation of spinal cord dysfunction by the somatosensory evoked potentials (SEPs) in dogs [Ph.D dissertation]. 2000. Seoul: Seoul National University.
22. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007. 8:275–282.

23. Ma WQ, Zhang SC, Li M, Yan YB, Ni CR. Experimental study of peripheral nerve grafts for repairing of chronic spinal cord injury in adult rats. Zhongguo Gu Shang. 2008. 21:519–521.
24. Mihai G, Nout YS, Tovar CA, Miller BA, Schmalbrock P, Bresnahan JC, Beattie MS. Longitudinal comparison of two severities of unilateral cervical spinal cord injury using magnetic resonance imaging in rats. J Neurotrauma. 2008. 25:1–18.

25. Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006. 24:376–385.

26. Muir WW 3rd, Wiese AJ, March PA. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am J Vet Res. 2003. 64:1155–1160.

27. Ogawa R. The importance of adipose-derived stem cells and vascularized tissue regeneration in the field of tissue transplantation. Curr Stem Cell Res Ther. 2006. 1:13–20.

28. Olby NJ, De Risio L, Munana KR, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res. 2001. 62:1624–1628.

29. Poncelet L, Michaux C, Balligand M. Study of spinal cord evoked injury potential by use of computer modeling and in dogs with naturally acquired thoracolumbar spinal cord compression. Am J Vet Res. 1998. 59:300–306.
30. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004. 109:1292–1298.

31. Rucker NC, Lumb WV, Scott RJ. Combined pharmacologic and surgical treatments for acute spinal cord trauma. Am J Vet Res. 1981. 42:1138–1142.

32. Safford KM, Rice HE. Stem cell therapy for neurologic disorders: therapeutic potential of adipose-derived stem cells. Curr Drug Targets. 2005. 6:57–62.

33. Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976). 2004. 29:1971–1979.

35. Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002. 5:438–445.

36. Vanický I, Urdzíkova L, Saganová K, Cízková D, Gálik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001. 18:1399–1407.

37. Webb AA, Jeffery ND, Olby NJ, Muir GD. Behavioural analysis of the efficacy of treatments for injuries to the spinal cord in animals. Vet Rec. 2004. 155:225–230.

38. Weber T, Vroemen M, Behr V, Neuberger T, Jakob P, Haase A, Schuierer G, Bogdahn U, Faber C, Weidner N. In vivo high-resolution MR imaging of neuropathologic changes in the injured rat spinal cord. AJNR Am J Neuroradiol. 2006. 27:598–604.
39. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.

40. Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS ONE. 2008. 3:e3336.

41. Yang JW, Jeong SM, Seo KM, Nam TC. Effects of corticosteroid and electroacupuncture on experimental spinal cord injury in dogs. J Vet Sci. 2003. 4:97–101.

42. Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia. 2005. 50:247–257.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download