Abstract
A not pregnant 4-year-old Jersey cow was presented with the sudden appearance of respiratory noise, nasal discharge and moderate respiratory difficulty. Upon physical examination a snoring-like noise, extended head and neck position, exaggerated abdominal effort, bilateral nasal discharge and left prescapular lymph node enlargement were noted. Sub-occlusion of the initial portion of the respiratory tract was suspected. Radiographic and endoscopic examinations revealed a pedunculate mass on the dorsal aspect of the rhinopharynx, which was removed with endoscopically assisted electrosurgery. Histologic examination revealed a chronic pyogranulomatous inflammation with eosinophilic club-like bodies surrounding small colonies of rod-shaped bacteria. Results of histochemical staining were consistent with Actinobacillus-like bacteria and a diagnosis of respiratory actinobacillosis was reached. Surgery and antibiotic therapy were resolutive, as demonstated by an endoscopic check at the second month after surgery, even without the association of the traditional iodine cure, which is regarded as the treatment of choice for actinobacillosis.
Actinobacillus (A.) lignieresii, a Gram-negative bacterium often found as a commensal in the upper digestive tract of cattle and sheep [1,4,7-10], may be responsible for the sporadic infection of soft tissues with regional lymph node involvement. In cattle, 'wooden tongue' is the classical presentation of this infection, in which soft tissue granulomas develop around the head, pharyngeal, chest, flank, stomach and limb regions [1,5,7,9,11].
The infection, which can also sometimes take on epidemic characteristics [2,3,5,6], develops following trauma that is capable of altering the integrity of the barrier of the oral mucosa [2] or the skin [5]. The cause is also sometimes of iatrogenic origin [3,9]. Besides these forms described in the literature, cases involving atypical locations have also been described in which actinobacillary lesions develop in various organs [10,11]. This short communication describes the clinical, radiological, endoscopic and pathological findings, and the surgical treatment of a case of atypical actinobacillosis in a cow.
A 4-year-old female Jersey bovine who was not pregnant, and had been born and raised at the Department of Veterinary Clinical Sciences, Faculty of Veterinary Medicine, University of Bologna, was examined following the sudden appearance of respiratory noise, nasal discharge and moderate respiratory difficulty.
Clinical examination demonstrated a snoring-like noise in the inspiratory phase, extended head and neck position, exaggerated abdominal effort and a dense, whitish mucous-like bilateral nasal discharge. Moreover, the left prescapular lymph node was moderately enlarged. Sub-occlusion of the initial portion of the respiratory tract was suspected.
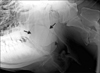
Hemochromocytometric and hematobiochemical analyses were unremarkable. X-ray examinations of the head, with a latero-lateral projection, showed the presence of a 10 cm-diameter mass at the rhinopharynx level (Fig. 1). Nasal endoscopy utilizing a model 780EG apparatus (Pentax Italia, Italy) was carried out after sedation with 0.025 mg/kg xylazine hydrochloride (Rompum; Bayer, Germany), 0.002 mg/kg butorphanol (Dolorex; Intervet-Schering Plough Animal Health, The Netherlands) and 1.5 mg/kg ketamine (Ketavet; Intervet-Schering Plough Animal Health, The Netherlands). A 5 × 10 cm reddish pedunculate mass covered with dense whitish mucous material was identified on the dorsal aspect of the rhinopharynx. Laryngeal access was partially obstructed during the inspiratory phase. Using a retroversion movement of the endoscope, it was possible to visualize the caudal portion of the mass from the laryngeal side. The mass displayed a rounded appendix. Radiographic and endoscopic results confirmed the clinical suspicion and the anatomic location of the lesion, but could not determine the nature of the neoformation, namely neoplastic or inflammatory, which, due to its position, required surgery.
Surgery was performed under sedation (as above) and truncal anesthesia of the two mandibular nerves by local infiltration of lidocaine hydrochloride as a 2% lidocaine solution (Azienda Terapeutica Italiana, Italy). Endoscopically assisted electrosurgery allowed partial remove of the mass, which was definitely resected by manual grasping through the oral cavity.
The defect was tamponed with iodopovidone (Betadine; Viatris, Italy), maintaining the head low to avoid aspiration. During the following three days the animal was treated with daily intramuscular injections of 30 mL benzylpenicillin and dihydrostreptomycin (Combiotic; Pfizer Animal Health, USA) and 1 mg/kg of flunixin meglumine (Finadyne; Shering Plough Animal Health,
The Netherlands).
The cow showed sudden remission of the respiratory symptoms. Moderate hyperthermia (39.5℃) was recorded only in first day after surgery. An endoscopic check in the second month after surgery showed a small thick scar of the mucosa and no recurrence.
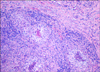
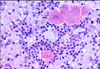
Histopathology of hematoxylin and eosin stained sections from formalin-fixed and paraffin-embedded samples demonstrated that the mass was composed of fibrous tissue and multiple confluent pyogranulomas containing small colonies of rod-shaped bacteria surrounded by eosinophilic club-like bodies (Figs. 2 and 3). Gram and Ziehl-Neelsen stains for acid-fast organisms were then carried out; results were Gram-negative and non-acid-fast, respectively. Accordingly, a histological diagnosis of chronic pyogranulomatous inflammation caused by Actinobacillus-like bacteria (probably A. lignieresii) was reached.
In this case, the respiratory symptoms manifested by the animal did not offer any particular difficulty in formulating a diagnosis of location, which was easily confirmed by endoscopy. The differential diagnosis for obstructive endopharyngeal diseases includes abscesses of the retropharyngeal lymph nodes, fibropapillomas, viral papillomas and infectious (tubercular or actinobacillar), allergic, mycotic or parasitic granulomas. Histopathology is the procedure of choice for their differentiation. Although definitive diagnosis relies on culture, the lesion pattern and the morphology of bacteria colonies were strongly suggestive of A. lignieresii infection, as their staining properties, size and shape excluded other organisms capable of causing granulomas (e.g. Mycobacteria spp.) or pyogranulomas (e.g. Actinomyces bovis, Staphylococcus aureus). As in granulomatous glossitis, the bacteria were putatively carried by a foreign body, probably a vegetal, although no remnants were evident histologically.
The endoscopic surgery represented a valid option for the removal of the mass associated with hemostasis, even if its position and the dimensions made a double surgical approach necessary.
Furthermore, the resolutive character of the surgical therapy, as demonstrated by the endoscopic check at the second month after surgery, is noteworthy since it was reached without the association of the "iodine cure" doctrinally described as the treatment of choice for actinobacillosis [12].
Figures and Tables
Fig. 1
Radiographic appearance of the head, latero-lateral projection. A mass is evident at the rhinopharynx level (arrows).
References
1. Aslani MR, Khodakaram A, Rezakhani A. An atypical case of actinobacillosis in a cow. J Vet Med A. 1995. 42:485–488.

2. Campbell SG, Whitlock TRH, Timoney JF, Underwood AM. An unusual epizootic of actinobacillosis in dairy heifers. J Am Vet Med Assoc. 1975. 166:604–606.
3. de Kruif A, Mijten P, Haesebrouck F, Hoorens J, Devriese L. Actinobacillosis in bovine caesarean sections. Vet Rec. 1992. 131:414–415.

5. Hebeler HF, Linton AH, Osborne AD. Atypical actinobacillosis in a dairy herd. Vet Rec. 1961. 73:517–521.
6. Julini M, Cravero G. Actinogranulomatosi linfonodale in vitelloni da carne. Prog Vet. 1979. 34:1151–1152.
7. Milne MH, Barrett DC, Mellor DJ, O'neill R, Fitzpatrick JL. Clinical recognition and treatment of bovine cutaneous actinobacillosis. Vet Rec. 2001. 148:273–274.

8. Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 2000. 9th ed. London: Saunders;909–944.
9. Rebhun WC, King JM, Hillman RB. Atypical actinobacillosis granulomas in cattle. Cornell Vet. 1988. 78:125–130.
10. Rycroft AN, Garside LH. Actinobacillus species and their role in animal disease. Vet J. 2000. 159:18–36.

11. Smith BP. Large Animal Internal Medicine. 2002. 3rd ed. St. Louis: Mosby;698–699.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download