Abstract
Twenty-five dogs were included in a randomized, double-blind trial to assess the efficacy of doxycycline (DOX) orally administered twice a day at 4 mg/kg/day (n = 12) for the treatment of osteoarthritis of the hip. Chondroitin sulfate (CS; 525 mg/day) was used as a positive control (n = 13). Dogs were re-examined monthly for 6 months after initiation of treatment. The assessment protocol included clinical score, radiographic findings and serum osteoarthritis biomarkers. Dogs treated with DOX showed statistically significant improvements (p < 0.05) in lameness, joint mobility, pain on palpation, weight-bearing and overall score at 2, 6, 4, 4 and 4 months, respectively, after treatment. Biomarker levels of CS-WF6 epitope and hyaluronan were significantly increased and decreased (p < 0.05) at 2 and 3 months after treatment compared to pretreatment. These results showed that DOX had a positive therapeutic effect in dogs with osteoarthritis.
Osteoarthritis (OA) is a chronic, disabling condition for which there are no cure and few useful treatments [9]. Clinical features include joint pain, instability, limitation of motion and function impairment. The pathogenesis of OA, albeit not yet well understood, is often linked to joint injury, biochemical alterations and ageing [20]. Pharmacological treatment alternatives for OA can be divided into two groups: symptom-modifying and disease-modifying drugs [7]. Symptom-modifying drugs are at present the prescription of choice for patients with OA. Drugs in this group are analgesics and non-steroidal anti-inflammatory drugs, which are effective in relieving the symptoms of OA [8]. There has recently been a lot of debate about some biological agents that are thought to have both symptom-modifying and disease-modifying properties [8,11].
Most of the compounds suggested as disease-modifying drugs are physiological molecules contained in articular tissues such as glucosamine sulfate and chondroitin sulfate (CS) [13]. A tetracycline antibiotic, doxycycline (DOX), has been successfully used to treat a wide-range of bacterial infections. In addition to its effects as an antibiotic, laboratory studies with animals and with human tissue have shown that doxycycline can inhibit the degradation of cartilage in a way that could be useful for the treatment of OA [24-26,38]. DOX, reportedly limits cartilage degradation and significantly ameliorates the degenerative changes that occur in OA joints [24-26,38]. Specifically, DOX orally administered at low dosages appeared to reduce the rate and extent of joint pathology in a canine model of OA [19,36,37]. However, most of the papers published about DOX and joint pathology were done in vitro [1,2,25,38]. Therefore, this study aims to investigate whether the long-term use of DOX can favorably modify the progression of OA in dogs. Moreover, to confirm preclinical data suggesting that DOX can slow the progression of OA. The experimental design was developed according to potential clinical use, with clinical score, radiographic findings and serum osteoarthritis biomarkers as primary outcome measures.
Twenty-five client-owned dogs were included in this study, 12 males and 13 females aged between 1~7 (4.21 ± 1.63) years old. Twenty dogs were Golden retrievers and 5 dogs were Labrador retrievers. Informed owner consent was obtained and the trial protocol was approved by the Faculty of Veterinary Medicine, Chiang Mai University's Ethics Committee, Chiang Mai, Thailand.
Golden and Labrador retriever dogs with clinical signs of chronic lameness, stiffness and joint pain and radiological evidence of OA of the hip were considered eligible for this study. Animals which were pregnant, receiving medication, or had hepatic, cardiovascular, gastrointestinal and neurological disease, were excluded. Dogs with lameness due to lumbosacral instability, infection, immune disease and fractures and dogs which previously received drug or dietary supplement for OA treatment were also excluded.
Dogs were clinically examined and blood samples were collected for baseline hematology, blood chemistry and biomarker for OA. Radiographs of hip joints were interpreted by two veterinarians.
The dogs were randomly assigned to two treatment groups. The first group (DOX group) received doxycycline (2 mg/kg body weight twice daily; Osoth Inter Laboratories, Thailand) [19], the second group (CS group) served as control group and received chondroitin sulfate (Fortiflex, 525 mg/dog daily; Virbac, USA). Animal were re-assessed monthly for clinical evaluation and blood collection, while radiographs were taken every 2 months. Treatment was stopped on the end of the 6th month.
Two veterinarians recorded the severity of the clinical signs at each monthly visit using an ordinal scoring system (Table 1) [10] and all veterinarians scored blind to the group classification. The radiographs of hip joints were taken every 2 months (3 times per animal) and were interpreted by the 2 veterinarians using the Takahashi scoring system (Table 2) [28]. Three milliliters of blood was collected monthly from the cephalic vein to assess the levels of OA biomarkers [16,17,22,23].
Efficacy of the treatment was determined by the mean of a clinical scoring system [10] that assessed the animal specific lameness, joint mobility, pain on palpation, weight-bearing and overall score of clinical condition. The dogs had to walk and trot 6 meters 3 times for the evaluation of lameness by 2 veterinarians, following palpation on hip joint for joint mobility and pain evaluation. The palpation was performed by 2 veterinarians, 30 min apart.
Structural joint changes were assessed on serial radiographs performed according to the standardized technique recommended by Takahashi [28]. Radiographs were taken for each animal at enrollment and at 3 and 6 months after treatment by the same technician using a usual X-ray machine (Kelex, Thailand). Ventrodorsal radiographs were obtained with the dog's hip and the leg in full extension position. Repositioning of the dog for subsequent radiographs were guided by the original film and the same radiographic setting (i.e. kilovolts, milliamperes and milliseconds) were used. All radiographs in a dog set (3 films) were interpreted for all evaluations concomitantly by 2 veterinarians using the criteria in Table 2.
Blood samples were analyzed for complete blood counts, including hematocrit, hemoglobin level, red blood cell count and white blood cell count and the platelet count. Two mililiters of serum were analyzed for aspartate aminotransferase, alanine aminotranferase, blood urea nitrogen and creatinine.
A mouse monoclonal antibody WF6 was raised against a shark cartilage aggrecan preparation and a quantitative ELISA for the epitope recognized by monoclonal antibody WF6 was modified from a previous study [16,22]. The antibody was specific for intact CS chains and showed no interaction with other sulfated glycosaminoglycans, hyaluronan or other polyanions, such as DNA, RNA or dextran sulfate. The standard used in the assay was shark cartilage aggrecan (A1 fraction) (Sigma-Aldrich, USA) at concentrations of 19~10,000 ng/mL in 6% Bovine serum albumin (BSA) in Tris Incubation (TI) buffer (0.1 M Tris HCl, pH 7.4 containing 0.15 M sodium chloride, 0.1% Tween 20 and 0.1% BSA). Diluted human serum samples (1 : 5 in 6% BSA-TI) were added to 1.5 mL plastic tubes containing an equal volume of WF6 (cell culture supernatant, 1 : 200 dilution in TI buffer). They were incubated at 37℃ for 1 h, and then added to the microtiter plate, which was pre-coated with shark aggrecan (A1 fraction). Non-specific protein binding was blocked with BSA. The plates were then incubated at 37℃ for 1 h, and the wells were then washed and peroxidase-conjugated anti-mouse IgM antibody (1 : 2,000) was added (100 mL/well; in TI buffer). The bound conjugate was detected by adding ortho-phenylenediamine (o-PD) substrate (100 mL/well in 0.05 M citrate buffer, pH 5.0). The reaction was stopped after 10 min with 50 mL/well of 4 M sulfuric acid, and absorbance was determined using a microplate reader (Titertek multiscan Mcc/340; ICN-Flow, USA) at 492/690 nm. The concentration of WF6 epitope in supernatant samples was calculated by reference to a standard curve.
Human serum samples or standard HA (HealonR; Pharmacia Pharmaceutical AB, Sweden) at various concentrations [19~10,000 ng/mL in 6% bovine serum albumin (BSA)-phosphate buffer saline (PBS) pH 7.4] were added to 1.5 mL plastic tubes containing biotinylated HABPs prepared as described above (1 : 200 in 0.05 M Tris-HCl buffer, pH 8.6). The tubes were incubated at room temperature for 1 h, and then samples were added to the microplate, which was precoated with umbilical cord HA (100 mL/well of 10 mg/mL) and blocked with 1% BSA (150 mL/well). The plate was then incubated at room temperature for 1 h. The wells were then washed and peroxidase-conjugated antibiotin antibody (1 : 2,000 dilution; Zymed, USA), 100 mL/well in PBS, was added. The plate was incubated at room temperature for another hour. The detection of conjugated antibody was with o-PD substrate and plate reading was carried out as described above. The concentration of HA in samples was calculated from the standard curve [16,23].
The results of CS and HA analyses are presented as mean ± SD. The non-parametric 2-sample Mann-Whitney procedure was used to test for differences between the DOX and CS groups. The radiograph and clinical sign scores were calculated as mean ± SD. The non-parametric 2-sample Mann-Whitney procedure was used to test for differences between the DOX and CS groups in the same month and between before and after treatment. The relative data was analyzed using the Statistical Analysis System version 8.0 (SAS Institute, USA) software package. p ≤ 0.05 was considered to be significant.
Thirty dogs were enrolled in the trial but 5 were withdrawn due the failure to attend an assessment appointment (2 dogs in the DOX group, 1 in the CS group) and death from a car accident (1 dog in the DOX group and 1 in the CS group). Table 3 shows the summary of age, sex and body weight data of 25 dogs completing the trial to the 6th month. All dogs enrolled in the trial had hemogram and biochemical profile results within reference range throughout the trial (6 months). Comparisons of pre-treatment disease score found no significant difference (p > 0.05) between the DOX and CS groups (Table 4).
Fig. 1 presents the 5 clinical score data before treatment (month 0) and at one month intervals until 6 months. The lameness score in the DOX group showed significant improvements (p < 0.05) at th..e 2nd month, while in the CS group it showed significant improvements (p < 0.05) in the first month. Joint mobility score in the DOX and CS groups showed significant improvements (p < 0.05) at the 6th and 4th months, respectively. Pain at palpation, weight bearing and the overall score in the DOX group showed significant improvements (p < 0.05) at the 4th month, but in the CS group it showed significant improvements (p < 0.05) earlier, in the 2nd month of treatment. Lameness, joint mobility, pain of palpation and overall score in the CS group was significant better (p > 0.05) than in the DOX group. The weight bearing score in the CS group was significant better (p > 0.05) than in the DOX group from the first month.
Radiography scores are shown in Fig. 2. Those scores were not significantly difference between treatment groups (p > 0.05). When comparing with the pretreatment scores, there also was no significant difference (p < 0.05).
The results of serum biomarkers for OA are shown in Fig. 3. The level of CS-WF6 epitope in the DOX and CS groups were significantly higher than before treatment. Differences between the 2 groups were significant in the first and second months after treatment. The level of HA in the DOX and CS groups were significantly higher than before treatment after 3 and 1 months of treatment, respectively.
The results of this study showed that dogs with OA had significant improvements in score for clinical evaluation and biomarker levels when treated with oral DOX. However, all of these effects occurred slower when compared to CS.
Disease-modifying or symptomatic slow-acting drugs are more interesting because some have been shown to be effective in improving symptoms and in reducing OA cartilage degradation with a reasonable safety profile [8,11]. These drugs have shown onset of efficacy and a prolonged residual effect once treatment is stopped [7]. As this study was a clinical trial, CS was chosen as positive control in order to address ethical responsibilities and the welfare of the participant dogs. According to previous studies, CS has been shown to reduce pro-inflammatory factors, modify the cellular death process and improve the anabolism/catabolism balance of extracellular cartilage matrix [12,13]. At the same time it has proven to have a positive effect on the pathological process involving the synovial tissue and subchondral bone. These mechanisms could, account for the beneficial results observed in some clinical trials [12,13,31].
The recognized limitation of this study was the lack of an objective assessment of the joint. It was not possible to perform ground force reaction measurements as was done in the trials of Hazewinkel [6], Moreau [14] and Vasseur [32] as this was a multicenter trial. Subjective assessment of weight bearing by 2 blinded veterinarians was used instead. Our study found that DOX had a slower effect on clinical improvements compare to CS. The overall score was improved 3 months after treatment, while CS had a significant effect 2 months after treatment. Moreover, we found that DOX did not improve the measures of pain as well as CS. Radiographic findings did not show any significant changes. In agreement with a published study [4], DOX did not significantly prevent the onset of progressive joint space narrowing (JSN) in the contralateral knee, and did not improve measures of pain or function of the OA knee.
The morphological changes in OA include alterations in the cartilage, subchondral bone, and synovial membrane [9,20]. Current knowledge points to an important involvement of the matrix metalloproteases (MMPs) class in the OA process [20]. Collagenase-3 (MMP-13) was demonstrated to play a major role in cartilage degeneration. It is also suggested that another enzyme, aggrecanase-2, or ADAMTS-5 plays a predominant role in the proteolysis of OA cartilage aggrecan [5,27].
It is recognized that MMPs play a role in the pathologic breakdown of the joint extracellular matrix in OA. It is known that low-dose regimens of a tetracycline analogue, namely DOX can inhibit some MMPs, hence reducing the extracellular matrix breakdown [1,2]. A recent study examined the effects of DOX on knee OA progression [4]. The primary outcome measure was JSN in the medial tibiofemoral compartment. Obese females with a unilateral OA knee were randomly assigned to receive 30 months of treatment with DOX or placebo. The loss of joint space width in the index knee in the DOX group was less than in the placebo group. This study showed that DOX can reduce the progression of established OA in this patient population. It provides the first proof of concept of the effectiveness of anti-MMP strategies for developing disease-modifying drugs.
Inhibition of the MMPs superfamily is a very logical objective in OA. Moreover, tetracyclines inhibit collagenase levels and nitric oxide production in vitro, thereby decreasing chondrocyte MMPs activity and increasing proteoglycan synthesis attenuating OA in animal models [1]. In agreement with a previous study, Pardy [19] showed DOX treatment conserved bone strain energy density at 72 weeks. Doxycycline had little effect on the degradation of superficial osseous tissue at 36 week after anterior cruciate ligament transection (ACLT); by 72 weeks, DOX in ACLT canine model limited subchondral bone loss within the first 3 mm of periarticular bone with established OA. Significant bone loss occurred in the deeper trabecular bone for all groups. Substantial architectural adaptation within deeper trabecular bone accompanied changes in mechanics in early and established OA. In 1991, Yu was done in vitro studies, and indicated that levels of neutral MMPs in OA cartilage are elevated and that doxycycline inhibits collagenolytic and gelatinolytic activity in extracts of OA cartilage [38]. However, before tetracycline and its analogues, or even MMP inhibitors, can be considered to be an effective treatment in preventing knee OA progression, further investigations are needed.
A novel monoclonal antibody CS-WF6, which recognizes a native epitope in CS chain [23], was elevated after treatment. The finding of elevated levels of serum CS-WF6 epitope after treated with both DOX and CS reflector to alteration the metabolism of the cartilage. In chronic OA, the level of CS-WF6 epitope is higher than normal because the native CS chain in cartilage was degraded and release into the blood system [16,17,23]. The elevation of CS-WF6 epitope in this study was shown both drugs induced the synthesis of CS-chain in cartilage lead to have more proportion of CS in the cartilage. This new CSs were source of the degradation process in OA joint which made a CS-WF6 epitope up-regulation. We found DOX had a slower effect on cartilage metabolism than CS. In this study, the level of CS-WF6 epitope was found to be significantly elevated after 1 and 2 months in CS and DOX groups, respectively.
In an inflammatory rat model of arthritis, it was demonstrated that serum HA levels correlated with the degree of synovitis and clinical arthritis [3]. HA plays the key role in immobilizing aggrecans in articular cartilage; this balances the tension and compressive resilience in the collagen network by its osmotic properties. Also, the HA levels were related to joint inflammation in humans [21]. Serum HA has been studied as a biomarker of disease progression, since significantly increased levels were reported in cases of rheumatoid arthritis and progressive osteoarthritis, compared to the normal population [3,21,23]. In our study, the HA levels were significantly decreased after 1 and 2 months in CS and DOX groups, respectively. This means that both DOX and CS decreased the level of inflammation in the joint. Compared between 2 groups, CS reduce inflammation significantly faster than DOX.
The one importance issue in using DOX as disease-modifying drug in OA is the microbial resistance. This trial did not study the effect of using a chronic, sub-antimicrobial dose of DOX on microbial resistance. However, many studies had proved that using sub-antimicrobial dose DOX (20 mg twice a day) had no effect on the microbial resistance [29,35]. Microbial studies have documented the lack of any antimicrobial effect on the normal flora, periodontal and/or opportunistic pathogens, or change in antibiotic susceptibilities following the use of a sub-antimicrobial dose of DOX up to 9 months in double-blinded, placebo controlled, multicentered studies [29]. These studies examined the effect, or lack of effect, of sub-antimicrobial dose DOX on the sub-gingival flora [29,30,35] and on antibiotic resistances within this flora [29] in a periodontitis population. Likewise, there was no detectible effect of a 9-month regimen of sub-antimicrobial dose of DOX on the intestinal flora of a periodontitis population cross-sectionally, relative to placebo control, or longitudinally within the sub-antimicrobial dose DOX treatment group [33]. In 2007 Walker and colleague [34] had reported that there was no evidence that exposure to DOX, 20 mg twice a day, resulted in cross- or multiantibiotic resistance. No evidence was present that the use of a sub-antimicrobial dose of DOX for a period of 24 months in a population of periodontally diseased osteopenic women exerted any detectible effect on the microbial flora as determined by total anaerobic counts and total counts for actinomyces and streptococci. There was no evidence that a sub-antimicrobial dose of DOX resulted in the colonization or overgrowth by periodontal and/or opportunistic pathogens. In our trial, we used 4 mg/kg DOX daily, which is a sub-antimicrobial dose (10 mg/kg daily) [15], related to all publications which mention above that possible is using 4 mg/kg DOX daily has no effect on the microbial resistance. However, all studies on microbial resistance had been done in humans not canine, so to fulfill this hypothesis, the sub-antimicrobial dose effect on microbial resistance needs to be done in canines in the future.
OA is, by far, the most common type of arthritis in human and animals encountered worldwide, yet the development of effective disease-modifying treatments has lagged behind that of other arthritides. Current challenges that need to be met are an ideal pharmacy by using novel knowledge of the biochemistry, molecular biology and imaging findings that stop progression of disease and recovery of the cartilage function. This study showed one of the drugs which can be used as an OA disease-modifying drug, even though the efficacy was not as great as the positive control. However, comparing the cost-benefit of DOX, we believe that DOX will be the disease-modifying drugs of choice for treated OA in dogs. Indeed, the results of the present study suggest that using DOX 4 mg/kg daily for 6 months had no effect on the liver and kidney functions. This drug can improve the clinical signs of OA in a dog within 4 months. Moreover, we showed orally administered DOX can alter the anabolism of the articular cartilage. This information may prove useful for using DOX as disease-modifying drug in clinical practice.
Figures and Tables
 | Fig. 1Mean scores for lameness, joint mobility, pain on palpation, weight bearing and overall. Black = DOX group; white = CS group. *Values were significantly different compare to month 0 within the groups (p < 0.05). †Values were significantly different between groups within the month (p < 0.05). |
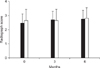 | Fig. 2Mean radiography scores. Black = DOX group; white = CS group. Vertical bar means a standard deviation. |
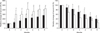 | Fig. 3Mean of relative change (%) of serum CS-WF6 epitope and hyaluronan (HA). Black = DOX group; white = CS group. *Values were significantly different compare to month 0 within the groups (p < 0.05). †Values were significantly different between groups within the month (p < 0.05). |
Acknowledgments
The authors would like to express their gratitude and thanks to all veterinarians and technicians at the Bone and Joint Research Laboratory, and the Small Animal Hospital, Faculty of Veterinary Medicine, Chiang Mai University for their kind support. This project was supported by The 2007 Young Researcher Grant, Chiang Mai University, Thailand and The National Research Council of Thailand (Research program of drug, chemical, medical material and equipment).
References
1. Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci USA. 1996. 93:14014–14019.

2. Attur MG, Patel RN, Patel PD, Abramson SB, Amin AR. Tetracycline up-regulates COX-2 expression and prostaglandin E2 production independent of its effect on nitric oxide. J Immunol. 1999. 162:3160–3167.
3. Björk J, Kleinau S, Tengblad A, Smedegård G. Elevated levels of serum hyaluronate and correlation with disease activity in experimental models of arthritis. Arthritis Rheum. 1989. 32:306–311.

4. Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, Wolfe F, Schnitzer TJ, Moreland LW, Manzi S, Bradley JD, Sharma L, Oddis CV, Hugenberg ST, Heck LW. Effects of doxycycline on progression of osteoarthritis: Results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005. 52:2015–2025.

5. Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005. 434:644–648.

6. Hazewinkel HA, van den Brom WE, Theyse LF, Pollmeier M, Hanson PD. Comparison of the effects of firocoxib, carprofen and vedaprofen in a sodium urate crystal induced synovitis model of arthritis in dogs. Res Vet Sci. 2008. 84:74–79.

7. Lequesne M, Brandt K, Bellamy N, Moskowitz R, Menkes CJ, Pelletier JP, Altman R. Guidelines for testing slow acting drugs in osteoarthritis. J Rheumatol Suppl. 1994. 41:65–71.
8. Maddison JE, Johnston KJ. Maddison JE, Page SW, Church D, editors. Nonsteroidal anti-inflammatory drugs and chondroprotective agents. Small Animal Clinical Pharmacology. 2002. London: Saunders;251–269.
9. Martel-Pelletier J, Lajeunesse D, Fahmi H, Tardif G, Pelletier JP. New thoughts on the pathophysiology of osteoarthritis: One more step toward new therapeutic targets. Curr Rheumatol Rep. 2006. 8:30–36.

10. McCarthy G, O'donovan J, Jones B, McAllister H, Seed M, Mooney C. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet J. 2007. 174:54–61.

11. McNamara PS, Johnston SA, Todhunter RJ. Slow-acting, disease-modifying osteoarthritis agents. Vet Clin North Am Small Anim Pract. 1997. 27:863–881.

12. Michel BA, Stucki G, Frey D, De Vathaire F, Vignon E, Bruehlmann P, Uebelhart D. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: a randomized, controlled trial. Arthritis Rheum. 2005. 52:779–786.

13. Monfort J, Pelletier JP, Garcia-Giralt N, Martel-Pelletier J. Biochemical basis of the effect of chondroitin sulphate on osteoarthritis articular tissues. Ann Rheum Dis. 2008. 67:735–740.

14. Moreau M, Dupuis J, Bonneau NH, Desnoyers M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003. 152:323–329.

15. Morgan RV. Handbook of Small Animal Practice. 2008. 5th ed. St. Louis: Saunders;1351.
16. Nganvongpanit K, Itthiarbha A, Ong-Chai S, Kongtawelert P. Evaluation of serum chondroitin sulfate and hyaluronan: biomarkers for osteoarthritis in canine hip dysplasia. J Vet Sci. 2008. 9:317–325.

17. Nganvongpanit K, Suwankong N, Jitpean S, Ong-Chai S. The changes of serum chondroitin sulfate in the induced osteoarthritic dogs after chitosan polysulfate administration. J Thai Vet Pract. 2005. 17:27–39.
18. Paimela L, Heiskanen A, Kurki P, Helve T, Leirisalo-Repo M. Serum hyaluronate level as a predictor of radiologic progression in early rheumatoid arthritis. Arthritis Rheum. 1991. 34:815–821.

19. Pardy CK, Matyas JR, Zernicke RF. Doxycycline effects on mechanical and morphometrical properties of early- and late-stage osteoarthritic bone following anterior cruciate ligament injury. J Appl Physiol. 2004. 97:1254–1260.

20. Pelletier JP, Martel-Pelletier J, Raynauld JP. Most recent developments in strategies to reduce the progression of structural changes in osteoarthritis: today and tomorrow. Arthritis Res Ther. 2006. 8:206.
21. Poole AR, Witter J, Roberts N, Piccolo F, Brandt R, Paquin J, Baron M. Inflammation and cartilage metabolism in rheumatoid arthritis. Studies of the blood markers hyaluronic acid, orosomucoid, and keratan sulfate. Arthritis Rheum. 1990. 33:790–799.

22. Pothacharoen P, Siriaunkgul S, Ong-Chai S, Supabandhu J, Kumja P, Wanaphirak C, Sugahara K, Hardingham T, Kongtawelert P. Raised serum chondroitin sulfate epitope level in ovarian epithelial cancer. J Biochem. 2006. 140:517–524.

23. Pothacharoen P, Teekachunhatean S, Louthrenoo W, Yingsung W, Ong-Chai S, Hardingham T, Kongtawelert P. Raised chondroitin sulfate epitopes and hyaluronan in serum from rheumatoid arthritis and osteoarthritis patients. Osteoarthritis Cartilage. 2006. 14:299–301.

24. Ryan ME, Greenwald RA, Golub LM. Potential of tetracyclines to modify cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1996. 8:238–247.

25. Smith GN Jr, Brandt KD, Hasty KA. Activation of recombinant human neutrophil procollagenase in the presence of doxycycline results in fragmentation of the enzyme and loss of enzyme activity. Arthritis Rheum. 1996. 39:235–244.

26. Smith GN Jr, Yu LP Jr, Brandt KD, Capello WN. Oral administration of doxycycline reduces collagenase and gelatinase activities in extracts of human osteoarthritic cartilage. J Rheumatol. 1998. 25:532–535.
27. Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005. 434:648–652.

28. Takahashi M, Naito K, Abe M, Sawada T, Nagano A. Relationship between radiographic grading of osteoarthritis and the biochemical markers for arthritis in knee osteoarthritis. Arthritis Res Ther. 2004. 6:R208–R212.
29. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000. 71:1472–1483.

30. Thomas JG, Metheny RJ, Karakiozis JM, Wetzel JM, Crout RJ. Long-term sub-antimicrobial doxycycline (Periostat) as adjunctive management in adult periodontitis: Effects on subgingival bacterial population dynamics. Adv Dent Res. 1998. 12:32–39.

31. Uebelhart D, Malaise M, Marcolongo R, de Vathaire F, Piperno M, Mailleux E, Fioravanti A, Matoso L, Vignon E. Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo. Osteoarthritis Cartilage. 2004. 12:269–276.

32. Vasseur PB, Johnson AL, Budsberg SC, Lincoln JD, Toombs JP, Whitehair JG, Lentz EL. Randomized, controlled trial of the efficacy of carprofen, a nonsteroidal anti-inflammatory drug, in the treatment of osteoarthritis in dogs. J Am Vet Med Assoc. 1995. 206:807–811.
33. Walker C, Preshaw PM, Novak J, Hefti AF, Bradshaw M, Powala C. Long-term treatment with sub-antimicrobial dose doxycycline has no antibacterial effect on intestinal flora. J Clin Periodontol. 2005. 32:1163–1169.

34. Walker C, Puumala S, Golub LM, Stoner JA, Reinhardt RA, Lee HM, Payne JB. Subantimicrobial Dose Doxycycline Effects on Osteopenic Bone Loss: Microbiologic Results. J Periodontol. 2007. 78:1590–1601.

35. Walker C, Thomas J, Nangó S, Lennon J, Wetzel J, Powala C. Long-term treatment with subantimicrobial dose doxycycline exerts no antibacterial effect on the subgingival microflora associated with adult periodontitis. J Periodontol. 2000. 71:1465–1471.

36. Yu LP Jr, Burr DB, Brandt KD, O'Connor BL, Rubinow A, Albrecht M. Effects of oral doxycycline administration on histomorphometry and dynamics of subchondral bone in a canine model of osteoarthritis. J Rheumatol. 1996. 23:137–142.
37. Yu LP Jr, Smith GN Jr, Brandt KD, Myers SL, O'Connor BL, Brandt DA. Reduction of the severity of canine osteoarthritis by prophylactic treatment with oral doxycycline. Arthritis Rheum. 1992. 35:1150–1159.

38. Yu LP Jr, Smith GN Jr, Hasty KA, Brandt KD. Doxycycline inhibits type XI collagenolytic activity of extracts from human osteoarthritic cartilage and of gelatinase. J Rheumatol. 1991. 18:1450–1452.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download