Abstract
Immunizing animals in the wild against Brucella (B.) abortus is essential to control bovine brucellosis because cattle can get the disease through close contact with infected wildlife. The aim of this experiment was to evaluate the effectiveness of the B. abortus strain RB51 vaccine in protecting infection as well as vertical transmission in Sprague-Dawley (SD) rats against B. abortus biotype 1. Virgin female SD rats (n = 48) two months of age were divided into two groups: one group (n = 24) received RB51 vaccine intraperitoneally with 3 × 1010 colony forming units (CFU) and the other group (n = 24) was used as non-vaccinated control. Non-vaccinated and RB51-vaccinated rats were challenged with 1.5 × 109 CFU of virulent B. abortus biotype 1 six weeks after vaccination. Three weeks after challenge, all rats were bred. Verification of RB51-vaccine induced protection in SD rats was determined by bacteriological, serological and molecular screening of maternal and fetal tissues at necropsy. The RB51 vaccine elicited 81.25% protection in SD rats against infection with B. abortus biotype 1. Offspring from rats vaccinated with RB51 had a decreased (p < 0.05) prevalence of vertical transmission of B. abortus biotype 1 compared to the offspring from non-vaccinated rats (20.23% and 87.50%, respectively). This is the first report of RB51 vaccination efficacy against the vertical transmission of B. abortus in the SD rat model.
Brucellosis is an economically important zoonotic disease that affects animals and humans. It is caused by the facultative intracellular bacteria belonged to the genus Brucella [23]. In most host species, the disease primarily affects the reproductive system [9], with major clinical manifestation of brucellosis in wildlife and cattle being abortion, decreased fertility, and placenta retention [27, 35]. Brucellosis has been known to exist in wildlife populations [10]. Wild rats are known to harbor Brucella organisms [17,36] and have found to be infected with Brucella (B.) abortus on farms where cattle were infected [17]. In Siberia and the Far East, gray rats were found to be a carrier of brucellosis [19]. The occurrence of disease in humans is largely dependent on the occurrence of brucellosis in wildlife reservoirs [12].
The transmission of B. abortus from dam to offspring has also been well documented in rats, mice, and cattle [2-4]. Vertical infection among offspring born to infected dams constitutes a major problem in eradication of brucellosis [34]. Successful eradication programs of brucellosis requires the elimination of brucellosis from the primary reservoir of the disease as well as free ranging wildlife, development of intervention measures against congenital brucellosis, use of efficacious vaccine and implementation of biosecurity measures [2,10,26].
B. abortus strain RB51 is widely used as a vaccine against bovine brucellosis in many countries, including the USA, in addition to the "test and slaughter" policy for the eradication of brucellosis in cattle [28]. RB51 is a stable rough mutant derived from the standard virulent B. abortus strain 2308 [28] and research data suggests that it induces protection in bison and cattle against abortion or infection with virulent B. abortus strains [20-22]. Our previous study had demonstrated vertical transmission of B. abortus biotype 1 in Sprague-Dawley (SD) rats [2]. In this study we verified the protective capacity of RB51 vaccine in the SD rats against infection as well as vertical transmission of the virulent B. abortus biotype 1 Korean bovine isolate.
Virgin female (n = 48) and male (n = 8) SD rats of 2 months of age, weighing 200~250 g were used for this experiment. The parent stocks of male and female rats were purchased from a government-licensed laboratory animal company (Koatech, Korea) which was bred to produce sufficient number of rats for use in this experiment. The rats were housed in a stringently hygienic, climate-controlled environment and supplied with commercial feed and water ad libitum. All experiments were carried out in compliance with the humane protocols approved by the Chonbuk National University, Jeonju, Korea under the supervision of veterinarians. The animals were culture negative for Brucella infection and seronegative for B. abortus antibodies, prior to the experimental infection, ascertained by routine bacteriological and serological tests.
Female virgin rats (n = 48) were divided into two groups: vaccinated group and non-vaccinated control group. The rats (n = 24) of the vaccinated group were injected intraperitoneally with 0.1 mL of physiological saline containing 3 × 1010 CFU of the B. abortus strain RB51 vaccine. The non-vaccinated control rats (n = 24) were injected intraperitoneally with 0.1 mL of sterile physiological saline solution. Both groups were challenged 6 weeks after vaccination with the virulent strain of B. abortus biotype 1 by an intraperitoneal injection of 1.5 × 109 CFU in 0.1 mL of normal saline. A master seed stock of RB51 was obtained from the Colorado Serum Company (USA). Before inoculation, the vaccine or challenge bacteria were cultured on brucella agar media (Difco, USA) for 7 days at 37℃ with 5% CO2.
All of the SD rats in our experiment were clinically examined after vaccination with RB51 or challenged with virulent B. abortus biotype 1. Rectal temperatures were recorded daily for the first week and the rats were observed twice daily for the duration of the study for adverse reactions or clinical signs such as anorexia, lethargy and anaphylaxis resulted from the vaccination or challenge.
Three weeks after challenge, RB51-vaccinated (n = 16) and non-vaccinated control (n = 16) female rats were simultaneously bred with healthy male SD rats at the ratio of 1 : 4 (1 male for 4 females). One male and four females were kept in a single cage for one month. Pregnant dams were placed in individual cages 3~4 days before parturition. After parturition the number of live or stillborn offspring/dam and weight of day-old live offspring were recorded.
Prior to the challenge and breeding, four randomly selected rats in RB51-vaccinated and non-vaccinated control groups were anesthetized by intraperitoneal administration of 15 mg/kg of tiletamine and zolazepam (Zoletil 50; Virbac Laboratories, France) and blood samples were collected by aseptic cardiac puncture. Necropsy of RB51-vaccinated and non-vaccinated rats were performed at 10 weeks after challenge with B. abortus biotype 1. Blood samples were collected from RB51-vaccinated and non-vaccinated parturient and non-pregnant rats under general anesthesia. The rats were then euthanized and spleen, liver, kidney, lung and uteri were collected. Day-old offspring of RB51-vaccinated and non-vaccinated rats were euthanized and spleen, liver and lung were collected. Serum samples were stored at -80℃ until tested. All other samples were stored at -20℃ until cultured.
All tissue samples were thawed. The tissue sample was macerated separately in a stomacher (IUL Instruments, Spain). Each of the macerated tissue samples was cultured in duplicate in blood agar media (Becton, Dickinson and Company, USA) and brucella agar media (Difco, USA) supplemented with antibiotics [Cycloheximide (100 mg/L), Polymixin B (25,000 IU/L), and Bacitracin (6,000 IU/L) that inhibit growth of bacteria other than Brucella] and incubated at 37℃ with 5% CO2 for 7 days. The identification of the isolates in the culture positive specimens was conducted by routine methods as described previously [1,28].
Bacteria harvested from culture positive specimens of rats were confirmed to be B. abortus biotype 1 by Bruce-ladder multiplex polymerase chain reaction (PCR) as described previously [16]. For Bruce-ladder multiplex PCR profiling, DNA was extracted from Brucella suspected colonies by a genomic DNA extraction kit (AccuPrep DNA Extraction Kit; Bioneer, Korea) using the manufacturer's protocol.
Thawed serum samples were screened for antibody to B. abortus biotype 1 by the Rose Bengal plate agglutination test (RBPAT) and standard tube agglutination test according to the previously described methods [1]. The total IgG, IgG1 and IgG2a titers in the sera of RB51-vaccinated and non-vaccinated control rats 3 weeks post challenge or at necropsy were measured by ELISA using smooth lipopolysaccharide (LPS) of B. abortus biotype 1. The LPS was extracted from the B. abortus biotype 1 by a commercial LPS extraction kit (Intron Biotechnology, Korea) using manufacturer's protocol. Flat-bottomed 96-well polystyrene microtiter plates (Nunc, Denmark) were coated with 100 µL of LPS (5 µg/mL) of B. abortus biotype 1 suspended in 0.05 mM sodium bicarbonate buffer (pH 9.6). Affinity purified rat IgG, IgG1 and IgG2a (Bethyl Laboratories, USA) were used to coat the 96-well plate starting from 500 ng/well to 7.8 ng/well to generate the standard curve. Each plate was incubated at 4℃ overnight. Plates were washed three times with wash solution [PBST: PBS (pH 7.4) with 0.05% (v/v) Tween 20]. Each well of the antigen-coated plates were blocked with 200 µL of blocking solution of 1% (w/v) bovine serum albumin (Sigma Aldrich, USA) in PBS and incubated at 37℃ for 30 min. Then the plates were washed three times with PBST. Each sample of sera was diluted 1 : 100 in sample diluent (50 mM tris, 0.14 M Nacl, 1% BSA, 0.05% Tween 20, pH 8.0) and 100 µL of diluted serum was added to duplicate wells of a 96-wells plate. The plates were sealed and incubated at 37℃ for 1 h. After five washing cycles with PBST, each well was incubated with 100 µL of 1 : 100,000 dilution of goat anti-rat IgG, IgG1 and IgG2a antibodies conjugated to horseradish peroxidase (Bethyl Laboratories, USA) diluted in conjugate diluent (50 mM tris, 0.14 M Nacl, 1% BSA, 0.05% Tween 20, pH 8.0), and the plates incubated at 37℃ for 1 h. After five washings as described above, the color reaction was developed by adding 200 µL/well of a solution containing 1.0 mg/mL of O-phenylenediamine dihydrochloride (OPD; Sigma, USA) in 0.05 M citrate buffer (pH 4.0) with 0.04% (v/v) H2O2. The plates were incubated in the dark for 30 min at room temperature then the colorimetric reaction was stopped by the addition of 50 µL/well of 3 M H2SO4. The absorbance measurements were made at 492 nm, using an automatic ELISA plate reader (Tecan, Austria).
The arithmetic means of offspring number, offspring weight and antibody titers between RB51-vaccinated and non-vaccinated control groups were compared for statistical significance by Student's t-test (Excel; Microsoft, USA). Chi-square analysis was used to compare vertical transmission rates between RB51-vaccinated and non-vaccinated control rats (Excel; Microsoft, USA). Significance of all the analyses was established at a p value of < 0.05.
The rectal temperature of rats after vaccination with B. abortus strain RB51 was within normal range (35~36℃). After challenge with virulent B. abortus, the non-vaccinated rats developed a fever, became lethargic and developed anorectic conditions within 24 h and the rectal temperature rose to 38℃ within 72 h. None of the RB51-vaccinated rats after virulent challenge manifested any abnormal clinical signs.
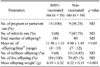
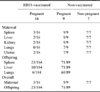
Significant protection against B. abortus infection was observed in RB51-vaccinated rats. All of the RB51-vaccinated rats (n = 16) became pregnant in spite of being intraperitoneally challenged with a field strain of B. abortus. On the other hand, 9 of 16 non-vaccinated challenged rats were pregnant. RB51-vaccinated rats delivered 184 live offspring while the 9 pregnant non-vaccinated control rats delivered 74 live and 15 stillborn offspring. No stillbirths were found in the RB51-vaccinated challenged rats. The average body weight of the day-old live offspring was 6.93 ± 0.32 g in RB51-vaccinated rats and 6.22 ± 0.31 g in non-vaccinated rats. The average litter size was 11.50 ± 1.31 for RB51-vaccinated rats and 9.88 ± 1.45 for non-vaccinated rats. Fertility rate was very low in non-vaccinated rats in comparison to RB51-vaccinated rats. The pregnancy rate, litter size, offspring weight and survival data are presented in Table 1.
Dam or offspring infection was defined as the recovery of the B. abortus biotype 1 challenge strain from any maternal or offspring sample. The B. abortus biotype 1 was recovered from several tissues at necropsy, though the recovery of B. abortus from samples was greater in the non-vaccinated group than the RB51-vaccinated group. The result of B. abortus biotype 1 recovery from necropsy samples is shown in Table 2.
All isolates recovered from the RB51-vaccinated and non-vaccinated challenged rats and offspring at necropsy were identified as smooth B. abortus by routine bacteriological methods such as crystal violet staining of the colonies, acriflavin agglutination, agglutination to anti-Brucella smooth and rough sera and biochemical tests (catalase, oxidase, nitrate reduction, urease). None of the isolates were identified as B. abortus strain RB51. All positive cultured bacterial colonies harvested from specimens of RB51-vaccinated and non-vaccinated female rats as well as offspring at necropsy were also confirmed as B. abortus biotype 1 by Bruce-ladder multiplex PCR assay with the predicted 1,682, 794, 587, 450 and 152-bp sized PCR amplicons (Fig. 1).
The B. abortus isolation rate was lower in RB51-vaccinated rats compared to non-vaccinated control female rats. At necropsy, all (n = 16) of the non-vaccinated challenged rats (100%) were culture positive to B. abortus. On the other hand, only 3 of 16 RB51-vaccinated challenged rats (18.75%) were culture positive to B. abortus. The rate of fetal infection or vertical transmission of B. abortus among offspring was significantly lower in RB51-vaccinated rats (12.50%) compared to the offspring born to non-vaccinated rats (79.77%) (p < 0.05). The overall results of bacterial isolation and identification from the RB51-vaccinated and non-vaccinated female SD rats and their offspring at necropsy are shown in Table 3.
Sera of four randomly selected rats in the RB51-vaccinated and non-vaccinated groups were seronegative prior to challenge by RBPAT, standard tube agglutination test and ELISA. On the day of breeding (3 weeks post-challenge), sera of four randomly selected rats from the RB51-vaccinated and non-vaccinated groups showed anti-B. abortus antibody responses in RBPAT, tube agglutination test and ELISA. The RB51-vaccinated rats had significantly lower (p < 0.05) standard tube agglutination titers at 3 weeks post-challenge when compared to non-vaccinated rats (Fig. 2). At necropsy, 3 of 16 RB51-vaccinated rats were seroconverted according to RBPAT, standard tube agglutination test and ELISA. All rats in the non-vaccinated group showed B. abortus specific antibody responses at necropsy and pregnant rats in this group had lower (p < 0.05) standard tube agglutination titers (314 ± 26) when compared to the non-pregnant rats (411 ± 11). The RB51-vaccinated rats had significantly lower (p < 0.001) standard tube agglutination titers (83 ± 14) when compared to non-vaccinated rats at necropsy (Fig. 2). Our ELISA data also showed significantly lower (p < 0.001) IgG, IgG1 and IgG2a titers in the sera of RB51-vaccinated rats compared to non-vaccinated control rats at 3 weeks post challenge or at necropsy (Fig. 3).
When antibody responses against LPS of B. abortus biotype 1 were evaluated by ELISA, total serum IgG, IgG1 and IgG2a titers were significantly lower (p < 0.001) in RB51-vaccinated rats (878 ± 13, 154 ± 3 and 185 ± 2 ng/mL, respectively) as compared to non-vaccinated control rats (1033 ± 16, 197 ± 3 and 242 ± 4 ng/mL, respectively) at 3 weeks after challenge with B. abortus biotype 1 (Fig. 3A).
At necropsy, total serum IgG, IgG1 and IgG2a titers measured by ELISA were significantly lower (p < 0.001) in RB51-vaccinated pregnant rats (509 ± 2, 35 ± 5 and 104 ± 0.2 ng/mL, respectively) in comparison to non-vaccinated control pregnant (1,284 ± 12, 324 ± 6 and 267 ± 2 ng/mL, respectively) and non-pregnant rats (1,320 ± 10, 357 ± 3 and 284 ± 2 ng/mL, respectively) (Fig. 3B).
Brucellosis has been emerging as a serious animal and public health problem in many parts of the world [8], including Korea [24], despite animal control and eradication programs. Control of brucellosis in animals is essential for its control in humans [13]. The current brucellosis control programs in many parts of the world, including Korea, are based on test and slaughter of sero-positive cattle [33]. If the slaughter of infected herds is limited to sero-positive adult animals, the latently infected calves could be a source of infection to the new farm [15]. Another likely source for reintroduction of brucellosis into livestock is from infected populations of free-ranging wildlife [10]. To control bovine brucellosis in endemic areas, the wild animals need to be free from brucellosis. Congenital infection caused by B. abortus in domesticated and wild animals is significant in the epizootiology of brucellosis [2,34] as eradication of brucellosis could not be achieved without preventing vertical transmission. The need to develop intervention measures against congenital brucellosis in wild and domesticated animals prompted us to evaluate the RB51 vaccine on the protection of infection and vertical transmission against B. abortus biotype 1 in a SD rat model.
It is well established in the literature that brucellosis protection is measured by a significant decrease in abortions or birth of weak offspring, and a significant decrease in colonization of vaccinated animals when compared to non-vaccinated animal control after challenge [11]. The data of this study demonstrated that the pregnancy rate was higher in RB51-vaccinated rats (100%) compared with non-vaccinated rats (56.25%), and the mean litter size was also significantly higher in RB51-vaccinated rats (11.50 ± 1.31) in comparison to the non-vaccinated rats (9.88 ± 1.45) (p > 0.05). In addition, the mean weight of day-old live offspring was significantly higher in RB51-vaccinated rats (6.93 ± 0.32) compare to non-vaccinated rats (6.22 ± 0.31) (p < 0.001). B. abortus causes loss in productivity of animals by abortion, infertility, decrease in milk production and birth of weak and dead calves through reproductive tract infection [9]. In this study, 2 of 16 (12.5%) uteri of RB51-vaccinated pregnant rats and 7 of 9 (77.78%) non-vaccinated pregnant rats were found to be infected with B. abortus 10 weeks after challenge. The significant difference of pregnancy rate, litter size and weight of offspring between RB51-vaccinated and non-vaccinated rats in our studies indicated that RB51-vaccine elicited significant protection against infection in vaccinated rats when compared to non-vaccinated rats.
Our study demonstrated that RB51-vaccine protected 81.25% of female rats against Brucella infection and proteced 87.50% of offspring against the vertical transmission of B. abortus. Some studies have reported that RB51 vaccine protected 87% of inoculated heifers against infection and protected 70% of calves against congenital infection after experimental challenged with virulent B. abortus [6,26].
The isolation of the challenge strain from different specimens was used as the criterion for brucellosis infection, as it is widely recognized to be the most definitive criterion for measuring the effect of brucella vaccination [26]. In the current study, isolation of B. abortus biotype 1 was performed from the spleen, liver, kidney, lung and uterus, as these are most frequently infected tissue in B. abortus infected animals [7]. At necropsy, we recorded a low level of persistence of B. abortus in the maternal tissues of RB51-vaccinated rats (18.75%) compared to the non-vaccinated rat (100%). Localization of B. abortus was primarily recorded in the lymphoid and uterine tissues of the non-vaccinated rat. Results of our bacteriological data suggest that vaccination with RB51 was efficacious in preventing intrauterine and fetal infection following exposure to virulent strain of B. abortus.
In vaccine trials, the dosages and strains of B. abortus that are used for challenge are important. An ideal situation is obtained when over 99% of challenge controls become infected [18]. In the present study, all of the non-vaccinated control rats were found to be infected with B. abortus biotype 1 following experimental challenge with 1.5 × 109 CFU of B. abortus biotype 1.
The results of serology using RBPAT, standard tube agglutination test and ELISA were negative from the day of vaccination through the day of challenge confirming the absence of serological interference from RB51 vaccination with the serological diagnostic tests for bovine brucellosis. The antibody response in smooth B. abortus is directed against LPS O-antigen, which could be detected by serological tests [5,7,29,31]. The lack of antibody response in RB51 vaccinates is most likely due to the absence of O-antigen [5,7,28,29]. After challenging with B. abortus biotype 1 all rats gave positive results in the serological tests used in this study.
Reports of vaccination in mice and cattle with RB51 indicate that it does not induce protective antibody responses against B. abortus strain 2308 [14,30,32]. Our serological tests did not detect antibody responses induced by strain RB51 6 weeks after vaccination. Antibody responses in the present study were detected only after challenge with B. abortus biotype 1. Serological data in this study showed low antibody titers in the RB51-vaccinated rats compared to non-vaccinated rats following challenge with B. abortus biotype 1. The low recovery rate of B. abortus biotype 1 in the vaccinated rats might have been responsible for the reduction of sero-converted antibody titers in RB51-vaccinated rats compared to non-vaccinated rats. Bison vaccinated with RB51 also showed lower standard tube agglutination titers as compared to non-vaccinated control following an experimental challenge with B. abortus 2308 [21]. In mice, RB51 vaccine caused no increase of antibody titers over non-vaccinated control following a challenge with B. abortus 2308 [14].
In this study, the difference of antibody titers between RB51-vaccinated and non-vaccinated rats was greater at 10 weeks compared to 3 weeks after challenge, which might have resulted from the induction of enhanced resistance of RB51 vaccine against B. abortus at 10 weeks compared to 3 weeks after challenge. Previous studies reported that RB51 vaccines induces enhanced resistance against B. abortus in mice at 7 weeks and in cattle between 10 and 12 weeks after vaccination [28,31].
Cell mediated immune (CMI) response is important in clearing the infection caused by the intracellular bacteria B. abortus [14,31]. The RB51 vaccine induces a good CMI response in cattle [31], and mice were reported to be protected by RB51 vaccine against B. abortus 2308 through CMI response [14,32]. The RB51 vaccine in mice and bison significantly reduced the recovery of virulent B. abortus 2308 compared to non-vaccinated controls [14,21]. In our study, B. abortus isolation rate was lower in the RB51-vaccinated group compared to the non-vaccinated control group, suggesting that the RB51 vaccine in SD rats might have played an important role for the intracellular killing of challenged bacteria in various organs (spleen, liver, kidney, lung and uterus) through CMI response.
In the current study, we chose the intraperitoneal route for inoculation of B. abortus biotype 1 to determine the effect of this route on vertical transmission as well as reproductive functions of SD rats. Our present study demonstrated vertical transmission as well as reproductive disorders in SD rat, while our previous study recorded only vertical transmission in SD rats when inoculated with B. abortus subcutaneously [2]. We vaccinated SD rats with RB51 through the intraperitoneal route because other studies showed that this route confers the best protection in mice against B. abortus compared to subcutaneous and oral routes [14,25]. Under field conditions, the intraperitoneal route may not be a good option for vaccinating wildlife. Further studies are required for the development of an effective vaccine delivery system for free ranging wildlife under field conditions. Our data suggests that the RB51 vaccine is effective in protecting SD rats against infection and vertical transmission of B. abortus. Therefore, RB51 vaccine would be beneficial in reducing the prevalence of B. abortus in the wild rat to facilitate eradication of brucellosis in humans and animals.
Figures and Tables
 | Fig. 1Bruce-ladder multiplex PCR assay. Lane 1: DNA molecular weight standard marker; Lane 2: bacterial DNA of non-vaccinated rat; Lane 3: bacterial DNA of RB51-vaccinated rat; Lane 4: bacterial DNA of offspring born to non-vaccinated dam; Lane 5: bacterial DNA of offspring born to RB51-vaccinated dam; Lane 6: positive control with DNA of Brucella (B.) abortus strain 1119-3; Lane 7: positive control with DNA of B. abortus strain RB51; Lane 8: negative control without DNA. |
 | Fig. 2Standard tube agglutination titers of rats 3 weeks after challenge with B. abortus biotype 1 and at necropsy. Antibody titers are reported as mean ± SE. Statistically significant differences among RB51-vaccinated, non-vaccinated pregnant and non-pregnant control rats are indicated by asterisks (*p < 0.05 and †p < 0.001). |
 | Fig. 3Total serum IgG, IgG1 and IgG2a levels of non-vaccinated control and RB51-vaccinated rats 3 weeks after challenge with B. abortus biotype 1 (A) and at necropsy (B). Antibody titers are reported as mean ± SE. Statistically significant difference among RB51-vaccinated, non-vaccinated pregnant and non-pregnant control rats are indicated by asterisks (*p < 0.05 and †p < 0.001). |
Table 1
Pregnancy rate, litter size, offspring body weight and survival data in RB51-vaccinated and non-vaccinated rats challenged with Brucella (B.) abortus biotype 1
References
1. Alton GG, Jones LM, Angus RD, Verger JM. Techniques for the Brucellosis Laboratory. 1988. Paris: Institut National de la Recherche Agronomique;17–136.
2. Baek BK, Lee BO, Hur J, Rahman MS, Lee SI, Kakoma I. Evaluation of the Sprague-Dawley rat as a model for vertical transmission of Brucella abortus. Can J Vet Res. 2005. 69:305–308.
3. Bosseray N. Mother to young transmission of Brucella abortus infection in mouse model. Ann Rech Vet. 1982. 13:341–349.
4. Catlin JE, Sheehan EJ. Transmission of bovine brucellosis from dam to offspring. J Am Vet Med Assoc. 1986. 188:867–869.
5. Cheville NF, Jensen AE, Halling SM, Tatum FM, Morfitt DC, Hennager SG, Frerichs WM, Schurig G. Bacterial survival, lymph node changes, and immunologic responses of cattle vaccinated with standard and mutant strains of Brucella abortus. Am J Vet Res. 1992. 53:1881–1888.
6. Cheville NF, Olsen SC, Jensen AE, Stevens MG, Palmer MV, Florance AM. Effects of age at vaccination on efficacy of Brucella abortus strain RB51 to protect cattle against brucellosis. Am J Vet Res. 1996. 57:1153–1156.
7. Cheville NF, Stevens MG, Jensen AE, Tatum FM, Halling SM. Immune responses and protection against infection and abortion in cattle experimentally vaccinated with mutant strains of Brucella abortus. Am J Vet Res. 1993. 54:1591–1597.
9. Cutler SJ, Whatmore AM, Commander NJ. Brucellosis-new aspects of an old disease. J Appl Microbiol. 2005. 98:1270–1281.
10. Davis DS, Elzer PH. Brucella vaccines in wildlife. Vet Microbiol. 2002. 90:533–544.
11. Elzer PH, Enright FM, Colby L, Hagius SD, Walker JV, Fatemi MB, Kopec JD, Beal VC Jr, Schurig GG. Protection against infection and abortion induced by virulent challenge exposure after oral vaccination of cattle with Brucella abortus strain RB51. Am J Vet Res. 1998. 59:1575–1578.
12. Godfroid J. Brucellosis in wildlife. Rev Sci Tech. 2002. 21:277–286.
13. Jiang X, Baldwin CL. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993. 61:124–134.

14. Jiménez De Bagüés MP, Elzer PH, Jones SM, Blasco JM, Enright FM, Schurig GG, Winter AJ. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994. 62:4990–4996.

15. Lapraik RD, Moffat R. Latent bovine brucellosis. Vet Rec. 1982. 111:578–579.
16. López-Goñi I, García-Yoldí D, Marin CM, de Miguel MJ, Muñoz PM, Blasco JM, Jacques I, Grayon M, Cloeckaert A, Ferreira AC, Cardoso R, Corrêa de Sá MI, Walravens K, Albert D, Garin-Bastuji B. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J Clin Microbiol. 2008. 46:3484–3487.

17. Moore CG, Schnurrenberger PR. A review of naturally occurring Brucella abortus infections in wild mammals. J Am Vet Med Assoc. 1981. 179:1105–1112.
18. Moriyón I, Grilló MJ, Monreal D, González D, Marín C, López-Góñi I, Mainar-Jaime RC, Moreno E, Blasco JM. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet Res. 2004. 35:1–38.

19. Ol'iakova NV, Antoniuk VI. The gray rat (Rattus norvegicus) as a carrier of infectious causative agents in Siberia and the Far East. Med Parazitol (Mosk). 1989. May-Jun. 73–77.
20. Olsen SC. Responses of adult cattle to vaccination with a reduced dose of Brucella abortus strain RB51. Res Vet Sci. 2000. 69:135–140.

21. Olsen SC, Jensen AE, Stoffregen WC, Palmer MV. Efficacy of calfhood vaccination with Brucella abortus strain RB51 in protecting bison against brucellosis. Res Vet Sci. 2003. 74:17–22.

22. Palmer MV, Olsen SC, Cheville NF. Safety and immunogenicity of Brucella abortus strain RB51 vaccine in pregnant cattle. Am J Vet Res. 1997. 58:472–477.
23. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005. 352:2325–2336.

24. Park MY, Lee CS, Choi YS, Park SJ, Lee JS, Lee HB. A sporadic outbreak of human brucellosis in Korea. J Korean Med Sci. 2005. 20:941–946.

25. Pasquali P, Rosanna A, Pistoia C, Petrucci P, Ciuchini F. Brucella abortus RB51 induces protection in mice orally infected with the virulent strain B. abortus 2308. Infect Immun. 2003. 71:2326–2330.

26. Poester FP, Gonçalves VSP, Paixão TA, Santos RL, Olsen SC, Schurig GG, Lage AP. Efficacy of strain RB51 vaccine in heifers against experimental brucellosis. Vaccine. 2006. 24:5327–5334.

27. Rhyan JC, Quinn WJ, Stackhouse LS, Henderson JJ, Ewalt DR, Payeur JB, Johnson M, Meagher M. Abortion caused by Brucella abortus biovar 1 in a free-ranging bison (Bison bison) from Yellowstone National Park. J Wildl Dis. 1994. 30:445–446.

28. Schurig GG, Roop RM II, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991. 28:171–188.

29. Stevens MG, Hennager SG, Olsen SC, Cheville NF. Serologic responses in diagnostic tests for brucellosis in cattle vaccinated with Brucella abortus 19 or RB51. J Clin Microbiol. 1994. 32:1065–1066.

30. Stevens MG, Olsen SC. Antibody responses to Brucella abortus 2308 in Cattle vaccinated with B. abortus RB51. Infect Immun. 1996. 64:1030–1034.

31. Stevens MG, Olsen SC, Cheville NF. Comparative analysis of immune responses in cattle vaccinated with Brucella abortus strain 19 or strain RB51. Vet Immunol Immunopathol. 1995. 44:223–235.

32. Stevens MG, Olsen SC, Pugh GW Jr, Brees D. Comparison of immune responses and resistance to brucellosis in mice vaccinated with Brucella abortus 19 or RB51. Infect Immun. 1995. 63:264–270.

33. Wee SH, Nam HM, Kim CH. Emergence of brucellosis in cattle in the Republic of Korea. Vet Rec. 2008. 162:556–557.

34. Wilesmith JW. The persistence of Brucella abortus infection in calves: a retrospective study of heavily infected herds. Vet Rec. 1978. 103:149–153.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download