Abstract
The presence of galectin-3 was immunohistochemically quantified in bovine intestines infected with paratuberculosis (Johne's disease) to determine whether galectin-3 was involved in the formation of granulation tissue associated with the disease. Mycobacterium avium subsp. paratuberculosis infection was histochemically confirmed using Ziehl-Neelsen staining and molecularly diagnosed through rpoB DNA sequencing. Galectin-3 was detected in the majority of inflammatory cells, possibly macrophages, in the granulomatous lesions within affected tissues, including the ileum. These findings suggest that galectin-3 is associated with the formation of chronic granulation tissues in bovine paratuberculosis, probably through cell adhesion and anti-apoptosis mechanisms.
Galectin-3 is a β-galactoside-binding animal lectin that contains carbohydrate-recognition domains and displays multiple related functions [2]. Extracellular galectin-3 mediates inflammation [1], whereas intracellular galectin-3 regulates cell growth and anti-apoptosis, and modulates cell adhesion, thus inducing cell migration [16]. Galectin-3 has been found in the cytoplasm of various cell types, including inflammatory cells such as macrophages, dendritic cells, mast cells, neutrophils, and eosinophils [14,15]. The expression of galectin-3 is known to be upregulated following certain bacterial [5] and parasitic infections [24]. In addition, it is accumulated in phagosomes containing mycobacterium during the course of an infection [3]. Here, we propose that galectin-3 is associated with the formation of granulomatous inflammatory lesions in chronic diseases including paratuberculosis.
Paratuberculosis, or Johne's disease, is a chronic, granulomatous enteritis found in wild and domestic ruminants. The causative agent is Mycobacterium avium subsp. paratuberculosis [18], which is a slow-growing facultative intracellular bacterium that persists within macrophages in the intestinal tract for several years before clinical onset [6]. The symptoms of clinical paratuberculosis are chronic diarrhea and progressive weight loss; subclinically infected animals may display decreased production [22]. The gross lesions are characterized by a segmental thickening of the intestine and mesenteric lymphadenopathy [18].
The aim of this study was to examine the immunohistochemical localization of galectin-3 in granulomatous intestines of bovines infected with paratuberculosis to determine whether galectin-3 was involved in the formation of granulation tissue in chronic disease. rpoB, encoding the ß subunit of RNA polymerase [4], PCR-plasmid TA cloning-sequencing for Mycobacterium species was used to diagnose the presence of paratuberculosis [27].
Tissue samples (n = 3) from the small intestines, mainly ileum, and mesenteric lymph nodes were obtained from cows at slaughter that were suspected to be infected with paratuberculosis. Tissue samples were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and processed for paraffin-embedding. Sections (5 µm thick) were cut in a Microtome (Leica, Germany), and were routinely examined with hematoxylin and eosin staining and with Ziehl-Neelsen staining for acid-fast bacilli [17]. As a control, bovine ileum without acid-fast bacilli staining was used.
For immunohistochemistry, 5-µm-thick sections of paraffin-embedded bovine ileum and mesenteric lymph nodes were deparaffinized using xylene and pure ethanol before exposed to citrate buffer (0.01 M, pH 6.0). Samples were then heated in a microwave oven for 3 min. All subsequent steps were performed at room temperature. The sections were treated with 0.3% hydrogen peroxide in methyl alcohol for 20 min to block endogenous peroxidase activity. After three washes in PBS, the sections were blocked with 10% normal goat serum (Vector ABC Elite Kit; Vector Laboratories, USA), diluted in PBS for 1 h, and then incubated with rat anti-galectin-3 antibody (1 : 5,000) for 1 h. The rat anti-galectin-3 monoclonal antibody (1 mg/mL) was purified from the supernatant of hybridoma cells (TIB-166; ATCC, USA). After three washes in PBS, the sections were incubated with biotinylated goat anti-rat IgG (1 : 100; Vector Laboratories, USA) for 45 min. After three washes in PBS, the sections were incubated with the avidin-biotin peroxidase complex (Vector Laboratories, USA), prepared according to the manufacturer's instructions, for 45 min. The peroxidase reaction was developed using a peroxidase substrate kit (Vector Laboratories, USA) according to the manufacturer's protocol. After the completion of color development, the sections were counterstained with hematoxylin (Sigma, USA) for 5 sec, washed in running tap water for 20 min, dehydrated through a graded ethanol series, and then cleared with xylene and mounted with Canada balsam (Sigma, USA). As a negative control, the primary antibody was omitted.
For the diagnosis of paratuberculosis from paraffin embedded tissues, the paraffin-embedded specimens were deparaffinized with xylene and pure ethanol. DNA was extracted using the previously described bead beaterphenol extraction method [10]. The DNA pellet obtained was used as a template for PCR. The rpoB PCR was carried out as described previously [10]. The PCR products obtained were electrophoresed in a 1.5% agarose gel and purified using a QIAEX II gel extraction kit (Qiagen, Germany). The purified PCR product (5-10 ng) was cloned using a TA cloning kit (Invitrogen, USA) according to the manufacturer's instructions. Three to 10 colonies of transformed Escherichia coli were picked in each reaction, cultured, and used to prepare plasmid DNA with a High Pure Plasmid Isolation Kit (Roche, Germany). The nucleotide sequences of the cloned rpoB DNAs were directly determined from the purified plasmid using M13 forward and reverse primers, which were supplied in the TA cloning kit, a 373A automatic sequencer, and a BigDye Terminator Cycle Sequencing kit (PE Applied Biosystems, UK). For the sequencing reaction, 60 ng of PCR-amplified DNA, 3.2 pmol of either the forward or the reverse primer, and 4 µL of BigDye Terminator RR mix (PE Applied Biosystems, UK) were mixed and adjusted to a final volume of 20 µL by adding distilled water. The reaction was run with 5% (vol/vol) dimethyl sulfoxide for 30 cycles of 15 sec at 95℃, 10 sec at 50℃, and 4 min at 60℃. Both strands were sequenced as a cross-check. The sequences determined (306 bp) were aligned and compared to sequences in GenBank by using the multiple-alignment algorithms in the MegAlign package (Windows version 3.12e; DNASTAR, USA).
All three cases showed typical granulation tissues in the intestines with a varying degree of inflammation. The histological findings in three cases were similar. Briefly, the lamina propria and submucosa of the small intestine was thickened due to the infiltration of inflammatory cells (Fig. 1A). The thickening of the mucosa was attributable to the accumulation of typical lymphocytes with condensed nuclei and macrophages with foamy, pale cytoplasm (Fig. 1B). Giant cells including more than two nuclei were occasionally found (Fig. 1B, arrowheads). An accumulation of multinucleated giant cells were also found in the mesenteric lymph nodes (Fig. 1C).
In the acid-fast stained tissue sections of the small intestine, the presence of acid-fast bacilli was confirmed due to its red coloration (Fig. 2A). Acid-fast bacilli were found in various cell types, including macrophages, in the lamina propria and submucosa of the small intestine. The lamina propria just below the epithelium of the villi was intensely stained red, suggesting that acid-fast bacilli were compacted in macrophages within this area (Fig. 2C). In the adjacent tissues of the acid-fast stained section, galectin-3 was strongly expressed in the round cells (typical of macrophages) in the lamina propria and submucosa of the intestines (Fig. 2B) and moderately expressed in the intestinal epithelium. The intense staining patterns of galectin-3 in the villous lamina propria (Fig. 2D) largely overlapped with the acid-fast staining (Fig. 2C).
The rpoB DNA (368 bp) was amplified from the small intestine sample, including ileum. However, due to weak amplification, PCR-direct sequencing was not possible. Therefore, the PCR products obtained were cloned into a TA plasmid for sequencing, and the inserted DNA sequences of the sample were compared with the sequences of 44 reference strains of mycobacteria and sequences in the GenBank database. The sequence showed 100% homology with Mycobacterium avium subsp. paratuberculosis (GenBank accession no. AE016958.1). Therefore, the cows were confirmed to be positive for Mycobacterium avium subsp. paratuberculosis.
This study confirms for the first time the accumulation of galectin-3-positive macrophages in bovine paratuberculosis-infected granulation tissues, particularly the ileum. Galectin-3 has long been known as an important mediator of macrophage activation [1,7] and postulated to play a critical role in phagocytosis by macrophages [23]. However, the precise mechanisms of how galectin-3 interrupts bacilli digestion by phagosomes within macrophages during paratuberculosis infection remains unknown and requires further study. In addition, galectin-3 has been known to be a major adhesion molecule [8] and involved in the trafficking of inflammatory leukocytes, including activated macrophages [1]. Based on these previous studies, we hypothesize that macrophages continuously accumulate in the granulation tissues.
Following the accumulation of macrophages in the granulation tissues of paratuberculosis, macrophages were found to be alive in the lesions even though they contained bacilli [6]. Bacilli-containing macrophages are thought to survive due to the anti-apoptotic characteristics of galectin-3 [16,21,25]. The molecular mechanism underlying this effect is cytochrome c release from the mitochondria [26], which possibly blocks cell death. In contrast to the increase of galectin-3 in macrophages during paratuberculosis, galectin-3 decreases in the intestinal epithelia of animals with intestinal bowel disease [19], suggesting that the expression of galectin-3 is cell type-dependent.
In addition to the immunological role of galectin-3 in inflammatory cells, galectin-3 has been found in the epithelia of the digestive and respiratory tracts [9,12,20] as well as the reproductive organs [11,13], where mucus is abundant. In the present study, we found galectin-3 localized in the intestinal epithelia, particularly colocalized with mucin. Since galectin-3 is one of the ligands for mucin [8], we postulate that galectin-3 is involved in the mucin-mediated protective mechanisms of epithelial cells.
In conclusion, we postulate that intracellular galectin-3 is involved in the protection of macrophages that contain bacilli, while extracellular galectin-3 is involved in the facilitation of inflammatory cell accumulation. Both machineries synergistically lead to chronic granulomatous bowel disease in bovine paratuberculosis.
Figures and Tables
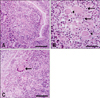 | Fig. 1Histopathologic findings of intestine and lymph nodes with with Johne's disease. (A) Accumulation of inflammatory cells in the lamina propria of the small intestine. (B) Higher magnification of (A) showing large macrophages (arrowheads) and lymphocytes (arrows) accumulated in the lamina propria. (C) Mesenteric lymph node. Note the multinucleated giant cell (arrow). A-C, H-E staining. A and C: Scale bars = 100 µm, B: Scale bar = 50 µm. |
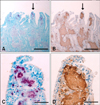 | Fig. 2Histochemical detection of acid-fast bacteria and immunohistochemical localization of galectin-3 in serial sections of small intestines infected with Johne's disease. Acid-fast bacilli were identified in the lamina propria of the small intestines using Ziehl-Neelsen staining (red color, A and C). Galectin-3 immunoreactivity (B and D) overlapped with acid-fast bacteria-containing cells (A) in the adjacent section. C and D show higher magnifications of arrow indicated fields in A and B, respectively. A and C: Ziehl-Neelsen staining. B and D: Immunostaining of galectin-3. A and B: Scale bars = 200 µm. C and D: Scale bars = 50 µm. |
References
2. Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994. 269:20807–20810.

3. Beatty WL, Rhoades ER, Hsu DK, Liu FT, Russell DG. Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell Microbiol. 2002. 4:167–176.

4. Boor KJ, Duncan ML, Price CW. Genetic and transcriptional organization of the region encoding the beta subunit of Bacillus subtilis RNA polymerase. J Biol Chem. 1995. 270:20329–20336.

5. Fowler M, Thomas RJ, Atherton J, Roberts IS, High NJ. Galectin-3 binds to Helicobacter pylori O-antigen: it is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell Microbiol. 2006. 8:44–54.

6. Harris NB, Barletta RG. Mycobacterium avium subsp. paratuberculosis in Veterinary Medicine. Clin Microbiol Rev. 2001. 14:489–512.

7. Hsu DK, Yang RY, Pan Z, Yu L, Samomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000. 156:1073–1083.

9. Kaltner H, Seyrek K, Heck A, Sinowatz F, Gabius H. Galectin-1 and galectin-3 in fetal development of bovine respiratory and digestive tracts. Comparison of cell type-specific expression profiles and subcellular localization. Cell Tissue Res. 2002. 307:35–46.

10. Kim BJ, Lee SH, Lyu MA, Kim SJ, Bai GH, Chae GT, Kim EC, Cha CY, Kook YH. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J Clin Microbiol. 1999. 37:1714–1720.

11. Kim H, Kang TY, Joo HG, Shin T. Immunohistochemical localization of galectin-3 in boar testis and epididymis. Acta Histochem. 2006. 108:481–485.

12. Kim H, Lee J, Hyun JW, Park JW, Joo HG, Shin T. Expression and immunohistochemical localization of galectin-3 in various mouse tissues. Cell Biol Int. 2007. 31:655–662.

13. Kim H, Ahn M, Moon C, Kim S, Jee Y, Joo HG, Shin T. Immunohistochemical study of galectin-3 in mature and immature bull testis and epididymis. J Vet Sci. 2008. 9:339–344.

15. Liu FT, Frigeri LG, Gritzmacher CA, Hsu DK, Robertson MW, Zuberi RI. Expression and function of an IgE-binding animal lectin (epsilon BP) in mast cells. Immunopharmacology. 1993. 26:187–195.

16. Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002. 1572:263–273.

17. Luna LG. Manual of Histologic Staining Methods of the Armed Forced Institute of Pathology. 1968. 3rd ed. New York: McGraw-Hill Book Company;220.
18. McGavin MD, Zachary JF. Pathologic Basis of Veterinary Disease. 2006. 4th ed. Philadelphia: Elsevier;372–374.
19. Müller S, Schaffer T, Flogerzi B, Fleetwood A, Weimann R, Schoepfer AM, Seibold F. Galectin-3 modulates T cell activity and is reduced in the inflamed intestinal epithelium in IBD. Inflamm Bowel Dis. 2006. 12:588–597.

20. Nio J, Kon Y, Iwanaga T. Differential cellular expression of galectin family mRNAs in the epithelial cells of the mouse digestive tract. J Histochem Cytochem. 2005. 53:1323–1334.

21. Oka N, Nakahara S, Takenaka Y, Fukumori T, Hogan V, Kanayama HO, Yanagawa T, Raz A. Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 2005. 65:7546–7553.

22. Olsen I, Sigurğardóttir G, Djønne B. Paratuberculosis with special reference to cattle. A review. Vet Q. 2002. 24:12–28.

23. Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003. 112:389–397.

24. Vray B, Camby I, Vercruysse V, Mijatovic T, Bovin NV, Ricciardi-Castagnoli P, Kaltner H, Salmon I, Gabius HJ, Kiss R. Up-regulation of galectin-3 and its ligands by Trypanosoma cruzi infection with modulation of adhesion and migration of murine dendritic cells. Glycobiology. 2004. 14:647–657.

25. Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996. 93:6737–6742.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download