Introduction
NO is induced during macrophage activation and thereby contributes to controlling the replication or neutralizing intracellular microbial pathogens [13]. Various studies indicated that NO is an important messenger in diverse biological functions, including neuronal transmission, vascular relaxation, immune modulation, and cytotoxicity against tumor cells [13,14].
β-glucans are heterogeneous groups of glucose polymers usually found in the cell walls of fungi [17], plants [11] and some bacteria [7]. They consist of linear β-1, 3-linked D-glucose molecules with β-1,6-linked side chains of varying length occurring at different intervals along the backbone, and can form complex tertiary structures stabilized by inter-chain hydrogen bonds [2,3].
Some animal studies addressed the beneficial effects of β-glucans on the growth performance of pigs [5,19], on the survival rate of mice challenged with Staphylococcus aureus or Candida albicans [16], and on the somatotropic axis and immune function in weaned piglets challenged with lipopolysaccharide (LPS) [12].
The problems associated with conventional methods of β-glucans extraction from mushrooms and plants, such as low purity and yield, high cost of production, as well as the adverse effects associated with intravenous administration β-glucans, such as inflammation, granuloma formation, and microembolization [18] prompted us to develop a more efficient method for extraction of extracellular β-(1→3), (1→6)-glucan from the soil based Paenibacillus (P.) polymyxa JB115 [7]. This study investigated the effects of β-glucans extracted from P. polymyxa JB115 on NO production in RAW264.7 murine macrophages.
In order to investigate the cytotoxicity of β-glucan on RAW264.7 macrophages, RAW264.7 cells (5 × 104 cells/ml) were incubated in a medium containing either β-glucan 30, 100 or 300 µg/ml or LPS (0.5 µg/ml) for 24 h. The viability of cells was then determined by MTT assay [8]. β-glucan decreased the viability of cells in a concentration-dependent manner (Fig. 1), with a statistically significant decrease (p < 0.05) being observed at a concentration of 300 µg/ml. LPS at 0.5 µg/ml also showed a significant decrease (p < 0.05) of approximately 60% relative to the control.
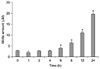
The effect of β-glucan on NO production in RAW264.7 macrophages was examined using a Griess reaction [4]. After 24 h of β-glucan exposure (30, 100 or 300 µg/ml), RAW264.7 cells showed a concentration-dependent production of NO (Fig. 2). This effect was also time dependent (Fig. 3).
Polysaccharides isolated form Phellinus linteus [8], Lentinus edodes [10] and Hericium erinaceum [20] are effective inducers of NO in macrophages. However, there have been other studies that demonstrated the inhibitory effect of β-glucans on macrophages stimulated by LPS or other factors [4,15]. In the present study, β-glucan from P. polymyxa JB115 activated RAW264.7 macrophages and induced the production of NO in a concentration- and time-dependent manner. However, this effect was not as potent as that of LPS (Figs. 2 and 3).
The cytotoxic effect of LPS in different cells including macrophages [21] and endothelial cells [6] has been well documented, and one of the most important factors associated with cell death is induction of NO [1,9]. These may also hold true in this study as the cytotoxicity of β-glucan may possibly be due to the NO production during macrophage activation.
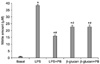
Polymyxin B has shown inhibitory effects on the lethal endotoxic activity of LPS in vivo and on the in vitro mitogenic activity of LPS by forming a stable molecular complex with the lipid A of LPS [21]. Therefore, this study also investigated the effects of polymyxin B on the activity of β-glucan and LPS in order to exclude any possible contamination due to endotoxins during the preparation process. Polymyxin B significantly (p < 0.05) inhibited NO production by LPS actvation. Nevertheless, polymyxin B had no significant effect on NO production induced by β-glucan (Fig. 4).
Finally, the mRNA expression of various cytokines was investigated in RAW264.7 macrophages which were exposed to β-glucan or LPS. P. polymyxa JB115 β-glucan induced mRNA expressions of i-NOS in a concentration-dependent manner, which might play a key role in NO production. A similar result was also observed for the mRNA expression of COX-2 and IL-6 (Fig. 5).
Based on our findings, we suggest further studies to be conducted to examine the potential use of the novel β-glucan purified from P. polymyxa JB115 as an immunostimulant or as an adjuvant of some animal vaccines.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download