Introduction
Neurodegenerative changes are morphologically characterized by progressive cell loss in vulnerable neuronal populations of the central nervous system (CNS), including specific regions of the hippocampus and specific subcortical regions. These changes are often associated with cytoskeletal protein aggregates that form intracytoplasmic and/or intranuclear inclusions in neurons and/or glial cells [14,27].
Aluminium (Al) is a neurotoxic metal that may be involved in the progression of neurodegenerative processes [9]. Entry of Al into the brain from the blood may involve transferrin-receptor mediated endocytosis and more rapid transport processing forsmall molecular weight Al species. There appears to be Al efflux from the brain, probably as Al citrate [43]. Several research groups have observed numerous ghost-like neurons with cytoplasmic and nuclear vacuolations and with Al deposits [21,28]. The hippocampus contained extracellular accumulations of Al and amyloids surrounded by nuclei of degenerating cells (neuritic plaques). Al promotes the formation and accumulation of insoluble beta amyloid (Abeta) and hyper-phosphorylated tau. In addition, Al mimics the deficient cortical cholinergic neurotransmission seen in several neurodegenerative diseases [43].
Reactive glial cell properties could contribute to the pathological mechanisms underlying neurodegenerative disturbances by favoring oxidative neuronal damage and Abeta toxicity [31]. Under conditions of neurodegeneration, excessive activation of microglia can contribute to the neurodegenerative process by releasing potentially cytotoxic substances, including the cytotoxic free radical nitric oxide (·NO) [17]. Chronic exposure to Al impairs glutamate-induced activation of nitric oxide synthase (NOS) and NO-induced activation of guanylate cyclase [8]. Cortical nitroxidergic neurons and granule cells are specific targets of Al neurotoxicity [29].
The NO-synthesizing enzyme NOS is present in the mammalian brain in 3 different isoforms: 2 constitutive enzymes (neuronal nNOS and endothelial eNOS) and 1 inducible enzyme (iNOS). All 3 isoforms are aberrantly expressed during Al intoxication. This gives rise to elevated levels of NO that are apparently involved in neurodegeneration by different mechanisms, including oxidative stress and activation of intracellular signaling mechanisms [19].
Al affects NO production in the brain. Al decreases glutamine synthase activity and also increases extracellular glutamate, which leads to developing the excitotoxic process that is under the influence of NO. Extracellular glutamate increases the influx of Ca, NOS activity and, consequently, increased NO synthesis [5].
Molecular oxygen (O2) is the primary biological electron acceptor that plays vital roles in fundamental cellular functions. However, concomitant with the beneficial properties of O2 comes the inadvertent formation of reactive oxygen species (ROS), such as superoxide anion radical (·O2-), hydrogen peroxide (H2O2) and hydroxyl radical (·HO) [30]. Accumulating evidence shows that iron (Fe) accumulates in the brain and catalyzes ·O2- formation, which reacts with NO to form the very toxic peroxynitrite anion (ONOO-) [20].
Free radicals (oxidative toxins) have been implicated in the destruction of cells via lipid peroxidative damage to cell membranes. After exposure to Al, increased malondialdehyde (MDA), an index of lipid peroxidation, was observed [35].
In normal cells, oxygen derivatives are neutralized or eliminated by a natural defense mechanism that involves enzymatic anti-oxidants (glutathione peroxidase, superoxide dismutase, catalase) and water or fat-soluble non-enzymatic anti-oxidants (vitamins C and E, glutathione, selenium) [32]. The antioxidant thiol L-gamma-Glutamyl-L-cysteinylglycine, or glutathione (GSH), has shaped, and is still refining, the nature of oxidative signaling in terms of regulating the milieu of inflammatory mediators, ostensibly via the modulation of oxygen- and redox-responsive transcription factors. Hence, they are termed redox(y)-sensitive cofactors [12]. Also, anti-oxidative defense includes both of the superoxide dismutase (SOD)-isoforms (Mn-SOD, Cu/Zn-SOD), which are induced to prevent oxidative and NO/ONOO- -mediated damage [25].
Cell death and changes in neurite morphology were partly reduced when the NO concentration was inhibited by NOS inhibitors [23]. Our previous results indicated positive effects of pre-treatment with NOS inhibitors on the development of neurotoxicity [33,38]. In view of the above considerations, the present study was undertaken to examine if the nitrite levels, ·O2- production, MDA concentrations, SOD activities and GSH contents that result after intracerebral injections of aluminium chloride (AlCl3) could be modulated by pretreatment with N-nitro-L-arginine methyl ester (L-NAME), a non-specific NOS inhibitor.
Materials and Methods
Animals
Male adult Wistar rats (500 ± 50 g) were used for these experiments. Animals were housed 2 or 3 per cage (Erath, Germany) in an air-conditioned room at a temperature of 23 ± 2℃, 55 ± 10% humidity and with lights on 12 h/d (07:00-19:00). The animals were given a commercial rat diet and tap water ad libitum. Animals used for these procedures were treated in strict accordance with the NIH Guide for Care and Use of Laboratory Animals (USA).
Chemicals
All chemicals were of analytical grade or better. AlCl3 was purchased from Sigma (USA); L-NAME was purchased from Sigma (USA); saline solution (0.9% w/v) was provided by the Hospital Pharmacy (Military Medical Academy, Serbia). All drug solutions were prepared on the day of the experiments.
Experimental procedures
The rats were anesthetized by intraperitoneal injection of pentobarbital sodium (45 mg/kg b.w.) before intrahippocampal administration of the following: (1) control group (n = 8) treated with 10 µl of 0.9% saline solution; (2) AlCl3 group (n = 15) - animals were treated with AlCl3 with 1 single dose (3.7 × 10-4 g/kg b.w. in 0.01 ml of deionizied water); (3) L-NAME + AlCl3 group (n = 10) - animals were pre-treated with L-NAME with 1 single dose (1 × 10-4 g dissolved in saline) before AlCl3 administration; (4) L-NAME group (n = 10) - animals were treated with L-NAME with 1 single dose (1 × 10-4 g dissolved in saline) before saline administration.
Using a stereotaxic instrument for small animals, chemicals were injected by Hamilton microsyringe into the CA1 sector of the hippocampus (coordinates: 2.5 A; 4.2 L; 2.4 V) [16]. L-NAME was immediately injected before the neurotoxin/saline solution. For all treated animals, the injected intracerebral volume was 10 µl and was always injected into the left side.
The 4 experimental groups groups (based on drug treatment) were subdivided into 2 subgroups each. At 2 time points after the treatments, 3 h and 30 d, animals in the subgroups were decapitated. Heads were immediately frozen in liquid nitrogen and stored at -70℃ until use. Crude mitochondrial fraction preparations of forebrain cotices were used for the biochemical analyses [11].
Biochemical analyses
After deproteinization, the production of NO was evaluated by measuring nitrite and nitrate concentrations. Nitrites were assayed directly by spectrophotometry at 492 nm using the colorimetric method of Griess (Griess reagent: 1.5% sulfanilamide in 1 M HCl plus 0.15% N-(1-naphthyl) ethylendiamine dihydrochloride in distilled water). Nitrates were first transformed into nitrites by cadmium reduction [24].
Superoxide anion (·O2-) content was determined by the reduction of nitroblue-tetrazolium (Merck, Germany) in the alkaline nitrogen saturated medium. Kinetic analysis was performed at 550 nm [2].
A lipid peroxidation index was determined as the quantity of malondialdehide (MDA) produced. Thiobarbituric acid (TBA) reagent (15% trichloroacetic acid + 0.375% TBA + 0.25% mol HCl) reacted with MDA, which was derived from polysaturated fatty acids during peroxidation. The reaction product, MDA, was measured spectrophotometrically at 533 nm [39].
SOD activity was measured spectrophotometrically as an inhibition of epinephrine spontaneous auto-oxidation at 480 nm. The kinetics of sample enzyme activity was followed in a carbonate buffer (50 mM, pH 10.2; Serva Feinbiochemica, Germany), which contained 0.1 mM EDTA (Sigma, USA), after the addition of 10 mM epinephrine (Sigma, USA) [34].
Content of reduced GSH was determined using 5,5-dithiobis-2-nitrobenzoic acid (DTNB, 36.9 mg in 10 ml of methanol), which had reacted with aliphatic thiol compounds in Tris-HCl buffer (0.4 mol, pH-8.9). This produced a yellow colored p-nitrophenol anion. Color intensity was used for spectrophotometric determination of GSH concentration at 412 nm. Brain tissue was prepared in 10% sulfosalicylic acid for GSH determination [1].
The protein content in the rat brain homogenates (forebrain cortex, ipsi- and contralateral) was measured by the Lowry method using bovine serum albumin (Sigma, USA) as a standard [18].
Results
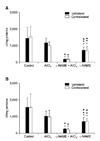
Nitrite levels in the rat forebrain cortex
The results in Fig. 1 show the bilateral nitrite levels (nM/mg proteins) in the rat forebrain cortex homogenates at 3 h (A) and 30 d (B) after the treatments. At the earlier test time, 3 h, AlCl3 injection resulted in increased nitrite production in the ipsilateral forebrain cortex that was significantly different compared to the control group (p < 0.05). Also, L-NAME + AlCl3 injection resulted in increased bilateral nitrite production in the forebrain cortex after 3 h compared to the control group. However, after 30 d, the L-NAME + AlCl3 injection resulted in lower nitrite levels compared to both the control and the AlCl3-treated groups (p < 0.05). At 3 h after L-NAME injection, nitrite production showed increased bilateral levels in the forebrain cortex compared to the control (p < 0.05). However, after 30 d, L-NAME injection resulted in lower nitrite levels in both the ipsi- and contralateral forebrain cortices compared to both the controls and the AlCl3-treated animals. After 30 d, L-NAME injection resulted in increased nitrite production in the ipsilateral forebrain cortex compared to the L-NAME + AlCl3-treated group (Fig. 1).
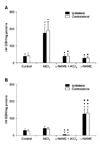
Superoxide anion radical production in the rat forebrain cortex
The results in Fig. 2 show the bilateral ·O2- levels (µM red NBT/min/mg proteins) in the rat forebrain cortex homogenates at 3 h (A) and 30 d (B) after the treatments. AlCl3 injection resulted in higher levels of ·O2- production after 3 h in the contralateral and after 30 d in both ipsi- and contralateral forebrain cortices compared to control animals (p < 0.05). In the L-NAME + AlCl3 group at 3 h, ·O2- production decreased bilaterally in the forebrain cortex compared to the AlCl3-treated group. Also, in the L-NAME + AlCl3 group at 30 d, ·O2- production decreased bilaterally in the same brain structure compared to both the control and the AlCl3-treated groups. At 3 h after L-NAME injection, ·O2- production was decreased in the ipsilateral forebrain cortex compared with controls, and bilaterally for this brain structure compared to the AlCl3-treated animals. At 30 d, NOS inhibitor injection resulted in lower bilateral ·O2- production in the forebrain cortex compared with controls, while higher ·O2- production was measured in both the ipsi- and contralateral forebrain cortices compared to the L-NAME + AlCl3-treated group (Fig. 2).
Malondialdehyde concentrations in the rat forebrain cortex
The results in Fig. 3 show the MDA concentrations (nM MDA/h/mg proteins) in the ipsi- and contralateral forebrain cortex homogenates at 3 h (A) and 30 d (B) after the treatments. For both test times (3 h, 30 d), AlCl3 injection resulted in increased bilateral MDA concentrations in the forebrain cortex that were significantly different compared to controls (p < 0.05). L-NAME + AlCl3 injection resulted in decreased MDA concentrations bilaterally in the same brain structure after 3 h and 30 d compared to the AlCl3-treated group. After 3 h, L-NAME injection resulted in a higher MDA concentration in the ipsilateral forebrain cortex compared to the control, and bilaterally in the same brain structure compared to the L-NAME + AlCl3-treated group. In contrast, lower MDA concentrations were measured in both the ipsi- and contralateral forebrain cortices compared to the AlCl3-treated group. After 30 d, L-NAME injection resulted in lower MDA bilateral concentrations in the forebrain cortex compared to both the control and the AlCl3-treated animals (Fig. 3).
Superoxide dismutase activities in the rat forebrain cortex
The results in Fig. 4 show the bilateral SOD activities (U/mg proteins) in the forebrain cortex homogenates at 3 h (A) and 30 d (B) after the treatments. At 3 h and 30 d after AlCl3 injection, there was lower SOD activity compared to the control group, although this was not significantly different. At 3 h and 30 d after L-NAME + AlCl3 injection, there was lower SOD activity compared to both the controls and the AlCl3-treated group (p < 0.05). At 3 h and 30 d, L-NAME injection resulted in lower bilateral SOD activity in the forebrain cortex compared to both controls and the AlCl3-treated animals. However, higher SOD activities were measured in both the ipsi- and contralateral forebrain cortices compared to the L-NAME + AlCl3-treated group (Fig. 4).
Reduced glutathione contents in the rat forebrain cortex
The results in Fig. 5 show the bilateral GSH contents (nM GSH/mg proteins) in the forebrain cortex homogenates at 3 h (A) and 30 d (B) after the treatments. AlCl3 injection resulted in higher bilateral GSH concentrations after 3 h in the forebrain cortex that was significantly different compared to the controls (p < 0.05). After 3 h, L-NAME + AlCl3 injection resulted in lower GSH contents in both the ipsi- and contralateral forebrain cortices compared to the AlCl3-treated group. Also, at 30 d after L-NAME + AlCl3 injection, GSH contents were decreased in both the ipsi- and contralateral forebrain cortices compared to both the control and the AlCl3-treated animals. After 3 h, L-NAME injection resulted in lower bilateral GSH contents in the forebrain cortex compared to the AlCl3-treated group. However, after 30 d, intrahippocampal L-NAME injection resulted in generally higher bilateral GSH contents in the forebrain cortex compared to the controls, as compared to the AlCl3-treated animals and as compared to the L-NAME + AlCl3-treated animals (Fig. 5).
Discussion
The injection of AlCl3 into the CA1 sector of the rat hippocampus resulted in significant increases in nitrite levels, ·O2- production, MDA concentrations and GSH contents in the forebrain cortex.
Numerous afferents from all areas of the cortex and the thalamus represent the most important sourcees of excitatory amino acids, whereas the nigrostriatal pathway and intrinsic circuits provide the striatum with dopamine, acetyl choline, GABA, NO and adenosine. Together, these neurotransmitter systems interact with each other and with voltage-dependent conductances to efficiently regulate synaptic transmission within this circuit [3].
In our study, AlCl3 injection resulted in increased nitrite concentrations after 3 h in the ipsilateral forebrain cortex, and bilateral nitrite concentrations were unchanged after 30 d in this brain structure compared to the controls. It has been previously shown [36] that production and oxidation of NO in the brain increased in the early stages of disease, while it decreased with increased loss of neurons.
The literature results implicate that the reaction product between NO and ·O2-, ONOO-, is a strong oxidizing and nitrating agent, which can react with all classes of biomolecules. In the CNS, this product can be generated by microglial cells that are activated by pro-inflammatory cytokines or Abeta and by neurons when ONOO- directly contributes to the initiation of the neurodegenerative process [40].
In our experiments, AlCl3 injection resulted in increased ·O2- production after 3 h in the contralateral forebrain cortex compared to controls. There are several lines of evidence showing that Al is associated with oxidative stress, possibly due to the pro-oxidant properties of Abeta present in senile plaques. The presence of low molecular weight Fe compounds can stimulate free radical production in the brain. Both Al and Abeta can potentate free radical formation by stabilizing iron in its more damaging ferrous (Fe2+) form, which can promote the Fenton reaction. The rate at which Fe2+ was spontaneously oxidized to Fe3+ was significantly lower in the presence of Al salts [42]. In addition, neurotoxin injection resulted in increased ·O2- production after 30 d in the ipsi- and contralateral forebrain cortices compared to controls. Recent results indicate that microglia are CNS macrophages and are primary cellular components of plaques that may contribute to the oxidative stress associated with chronic neurodegeneration [7].
Al has been shown to alter Ca2+ flux and homeostasis and to facilitate the peroxidation of membrane lipids. Literature results suggest that Al may facilitate increases in intracellular Ca2+ and ROS, and potentially contribute to neurotoxicity induced by other neurotoxicants [22]. We have shown that at all test times (3 h, 30 d) post AlCl3 injection, there were increased bilateral MDA concentrations in the forebrain cortex compared to the control animals.
Furthermore, we have shown that AlCl3 injection resulted in increased bilateral GSH concentrations after 3 h in the forebrain cortex compared to controls. Literature results implicate that all detrimental effects of ONOO- could be successfully attenuated by the thiol-containing anti-oxidant tripeptide glutathione [12]. Research shows that ONOO-can oxidatively modify both membranous and cytosolic proteins, which alters both their physical and chemical properties [15].
It has been previously shown [37] that the interactions between basalocortical and intracortical NOS neurons are involved in the spatial and temporal regulation of cortical perfusion following basal forebrain activation. Aluminium accumulation in the brain can alter neuronal signal transduction pathways associated with glutamate receptors. Impairment of the Glu-NO-cGMP pathway in the brain may be responsible for some of the neurological alterations that are induced by Al [4].
Decreased bilateral nitrite concentrations in the striatum at 30 d in the L-NAME + AlCl3 group, compared to the AlCl3-treated group, suggested that L-NAME suppressed nitrite production and decreased neuron impairment via N-methyl-D-aspartic acid (NMDA) receptors. Neuropharmacological data indicate that Abeta toxicity is mediated by an excitotoxic cascade involving blockade of astroglial glutamate uptake, sustained activation of NMDA receptors and an overt intracellular Ca2+ influx. These changes are associated with increased NOS activity in cortical target areas that may directly lead to the generation of free radicals [13]. A sustained overproduction of NO via NOS expression may be responsible, at least in part, for some of the neurodegenerative changes caused by stress and support a possible neuroprotective role for NOS inhibitors in this context [26].
Decreased bilateral ·O2- production in conjunction with decreased MDA concentration, as well as decreased bilateral SOD activity, in the forebrain cortex 3 h and 30 d after L-NAME + AlCl3 injection, compared to AlCl3-treated animals, suggested reduced enzyme substrate (·O2-) production. In the same experimental group of animals, we showed decreased GSH contents. Our results suggest the importance of GSH participation in the glutathionylation process as a crucial anti-oxidative defense mechanism against irreversible protein impairment [10].
Under our experimental conditions, 3 h after L-NAME injection, there was increased bilateral NO production in the forebrain cortex compared to the control group. Literature results suggest that the inhibition of inducible NOS expression by L-NAME administration prevented an increase in nitrogen intermediates and GABA release, but not in glutamate release [41]. Also, recent results indicate that, in some circumstances, L-NAME may contribute to NO donation by serving as an arginine analog [6]. In contrast, decreased NO concentrations, as well as decreased ·O2- production, 30 d after L-NAME injection suggests a long-term NO synthesis inhibition by L-NAME, in addition to potential L-NAME anti-oxidative effects.
Decreased bilateral SOD activities along with decreased GSH concentrations in the forebrain cortex post L-NAME injection contributed to oxidative development at the early time point. Thirty days post L-NAME injection showed improvements of an oxidative stress development parameter (increased GSH concentration). As a potential arginine analog in the specified experimental groups, L-NAME could overlap the toxic effects of AlCl3.
At 3 h after L-NAME injection, there was a higher MDA concentration in the ipsilateral forebrain cortex compared to controls, while a lower MDA concentration was measured bilaterally in the forebrain cortex at 30 d post L-NAME application. The analysis of MDA concentration is a crucial parameter of membrane lipid destruction that demonstrates a protective effect for L-NAME in AlCl3-treated animals in contrast to AlCl3- and L-NAME- effects.
Glutamate excitotoxicity, oxidative stress and mitochondrial dysfunctions are common features that lead to neuronal death after Al intoxication. Nitric oxide alone, or in cooperation with ·O2- and ONOO-, is emerging as a predominant effector of neurodegeneration [5].
The present results revealed that NO-mediated neurotoxicity resulting from intrahippocampal aluminium injection spread temporally and spatially in the forebrain cortex, and suggested a potential neuroprotective effect for L-NAME.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download