Abstract
This report deals with the acute onset of an abortion outbreak and high sow mortality in one pig herd consisted of 1,200 pigs and 120 sows on Jeju Island, Korea. Affected pregnant sows showed clinical signs, including high fever, gradual anorexia, vomiting, depression, recumbency, prostration, abortion, and a few deaths. Four dead sows, five aborted fetuses from the same litter, and 17 sera collected from sows infected or normal were submitted to the Pathology Division of the National Veterinary Research and Quarantine Service for diagnostic investigation. Grossly, hepatomegaly and splenomegaly were observed in sows. Multiple necrotic foci were scattered in the lungs, liver, spleen, and lymph nodes. Microscopically, multifocal necrotizing lesions and protozoan tachyzoites were present in the lesions. Tachyzoites of Toxoplasma (T.) gondii were detected immunohistochemically. Latex agglutination showed that the sera of 7 of 17 (41.2%) sows were positive for antibody to T. gondii. The disease outbreak in this herd was diagnosed as epizootic toxoplasmosis. To our knowledge, this is the first report of porcine toxoplasmosis with a high abortion rate and sow mortality in Korea.
Toxoplasmosis is caused by infection with Toxoplasma (T.) gondii, a coccidian parasite that can infect humans and animals [2,6,15]. Postnatally, animals and humans generally become infected after ingesting food and water contaminated with sporulated oocysts or by consuming raw or undercooked meat containing tissue cysts [2,7]. Although toxoplasmosis is generally asymptomatic, primary infections in pregnant women and animals may cause abortions, fetal abnormalities, or perinatal death [2,6].
Most T. gondii infections in pigs are subclinical [2], and transplacental infections are less common than post-natal infections [2]. Although abortions related to T. gondii are uncommon, they may occur in sows infected during pregnancy [2]. Pigs infected transplacentally may be born premature, dead, or weak, or they may die soon after birth [15].
In Korea, T. gondii infections have been reported in humans [20] and many domestic animals, including cats [5], dogs [9], and pigs [19]. To our knowledge, however, there have been no reports of abortions in animals related to this parasite in Korea. Herein we describe an abortion outbreak due to T. gondii infection in sows.
During September 2002, an outbreak of abortion, lasting 10 days, was observed in one pig herd in Jeju Island, Korea. The index herd was a 120-mixed breed-sow feeder pig producer herd located in Hallym County, in the western part of Jeju Island. This pig farm was isolated from other pig farms and the herd was housed in 2 separate pens. Weaned piglets were housed in the first pen. The second pen was divided into 4 rooms, which housed delivered sows, pregnant sows, fattening pigs, and grower pigs in rooms 1-4, respectively. The herd was maintained using a continuous flow system and had been routinely vaccinated for Japanese encephalitis virus, porcine parvovirus, and a few bacterial respiratory and enteric pathogens such as Bordetella bronchiseptica, Pasteurella multocida, Erysipelothrix rhusiopathiae, and Escherichia coli. One month before the disease outbreak, the source of commercial feed was changed. Water supply was from a private well. Three dogs were also present at the farm. There were five pig farms located within a 100 m radius.
At the time of the outbreak, the second room of the second pen housed 84 pregnant sows. Two weeks after changing the feed, 10 sows, especially first parity sows, exhibited poor appetite and vomiting. An abnormal stink was present in the new feed without unusual gross appearance at that time. Among the clinical signs observed in the 37 affected pregnant sows were fever (temperature of > 37℃), gradual anorexia, vomiting, depression, recumbency, prostration, and abortion. Sixteen sows died within 7 days of the initial manifestation of symptoms. Abortion usually occurred 3-5 days after the onset of clinical signs, and at any stage of gestation. The abortion rate was high (44%), and the mortality rate of the sows was 19%. Intensive antibiotics including neomycin, gentamicin and cefazolin and symptomatic therapies had no effect.
Two dead sows, five aborted fetuses from the same litter, and sera collected from 12 sows that had aborted and from 5 normal sows were submitted to the Pathology Division of the National Veterinary Research and Quarantine Service (NVRQS) for diagnostic investigation. Later additional internal tissues from two aborted sows obtained in the middle of culling were also submitted to the NVRQS.
Grossly, the four sows had cutaneous cyanosis in the ears, snout, and ventral abdomen. The submandibular and mediastinal lymph nodes were enlarged and bright red in color. The lungs did not fully collapse and exhibited pinpoint yellowish white foci throughout the whole lobes. Mild to moderate hepatomegaly and splenomegaly were also observed. Pale, white, dry miliary foci were scattered in the liver, spleen, and lymph nodes. The length from crown to rump of the fetuses ranged from 22 to 24 cm. No significant gross abnormalities were noted in any of the aborted fetuses.
Tissue samples from the lungs, heart, liver, kidneys, spleen, stomach, intestine, lymph nodes, and brain of sows and fetuses were fixed in 10% phosphate-buffered formalin, dehydrated, embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin for light microscopic examination.
Immunohistochemical assays for T. gondii were performed on the replicated paraffin sections of the lungs, liver and lymph nodes, as described previously [9,19]. Sections were mounted on Probe-On slides (Fisher, USA) and incubated with the primary antibody, unlabelled rabbit polyclonal antibody (Elite, USA) against T. gondii. The presence of antigen was determined using standard avidinbiotin-peroxidase complex (ABC) methods, according to the manufacturer's protocol (Elite, USA), with 3, 3-diaminobenzidine (Elite, USA) as the chromogen. Control procedures included omission of the primary antibody and substitution of an isotype-matched irrelevant antibody.
Samples were assayed for the presence of classical swine fever (CSF) virus, Aujeszky's disease (AD), porcine reproductive and respiratory syndrome virus (PRRSV), and porcine circovirus type 2 (PCV-2) using PCR, as described previously [10,17]. Reverse transcriptase-PCR for CSF virus was conducted on tissue homogenates from the sow to amplify the 5' untranslated region and E2 envelope glycoprotein gene [1]. Virus was isolated to exclude infection with AD infection.
Indirect fluorescent antibody (FA) tests for CSF, AD, PRRSV, and PCV-2 were performed on the replicated cryosections of the tonsils and lungs as described previously [8,10]. Cryo-sections were fixed in cold acetone for 5 min and incubated with primary monoclonal or polyclonal antibody against each pathogen, followed by fluorescein isothiocyanate-conjugated secondary antibody (Dakocytomation, Denmark) directed against the primary antibody.
Aseptically collected tissue samples from the lungs, liver, spleen, lymph nodes, and intestine were cultured on blood agar, MacConkey agar (BBL, USA), and Chocolate agar (Hanil Komed, Korea) at 37℃ under aerobic and anaerobic conditions.
Antibody titers to T. gondii were determined using a commercial latex agglutination (LA) test kit (Eiken Chemical, Japan) [11]. The reactions were performed in 96-well U-bottom polystyrene microplates (BD Biosciences, USA) at two-fold dilutions. To each well 25 µl of T. gondii antigen-coated latex particles suspension was added, followed by incubation overnight at room temperature. An agglutination titer ≥ 1 : 32 was considered positive. The serum antibody titers to CSF virus and AD virus were also screened by ELISA methods as described previously [17]. An indirect FA test to determine the antibody titers for Neospora (N.) caninum was performed as previously described [11].
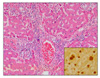
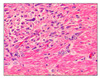
Microscopically, moderate multifocal necrotizing pneumonia with intralesional protozoan tachyzoites was present in the lungs of sows. Mild perivascular or peribronchiolar lymphoid cuffings associated with mycoplasmal pneumonia were observed in two sows. Severe multifocal necrotizing granulomatous cholangiohepatitis, characterized by the infiltration of lymphocytes, plasma cells, macrophages and a few neutrophils and protozoan tachyzoites, were observed in the livers (Fig. 1). The lymph nodes presented with severe lymphadenitis, with focal to diffuse necrosis and protozoan tachyzoites, and the spleens showed moderate congestion, lymphoid depletion and multifocal necrosis. Lymphohistiocytic meningoencephalitis with disseminated malacic foci and perivascular cuffing was observed in the brains (Fig. 2).
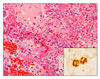
Three of five aborted fetuses showed multifocal necrotic lesions and intralesional or adjacent protozoan tachyzoites in the lungs (Fig. 3), liver, lymph nodes, and brain. In addition, the brains showed necrotic encephalitis, gliosis, and perivascular cuffing.
Sows and fetuses were negative for CSF, AD, PRRSV, and PCV-2 by PCR, virus isolation and FA tests. No specific bacterial pathogens were isolated, except for Pasteurella multocida, which was isolated from one sow with pneumonic consolidation.
At the time of submission, 7 of 17 (41.2%) selected sows were positive for T. gondii by an LA test and were judged to have been exposed to the parasite. LA titers of seropositive sows ranged from 1 : 32 (4), 1 : 64 (2), to 1 : 128 (1), respectively. All seropositive sows experienced recent abortions. The five normal sows without abortion were seronegative for T. gondii. Only one sow in the herd was serologically positive for CSF virus by ELISA, whereas none was serologically positive for AD virus and N. caninum.
Clinical signs and pathological changes may be nonspecific, requiring full panels of microbiological and serological tests to confirm the exact etiology. Diagnostic tests were able to rule out major endemic swine infectious diseases in Korea, including CSF, AD, PRRS, and PCV-2. Histopathologic examination of the major parenchymal organs of sows and aborted fetuses revealed many necrotic lesions associated with protozoan tachyzoites. Using T. gondii specific antibody, the presence of T. gondii tachyzoites was demonstrated in the formalin-fixed tissues from sows and fetuses. In addition, serological tests revealed high antibody titers to T. gondii in aborted sows from this herd. Based on these clinical signs, histopathology, immunohistochemistry and microbiology, the herd was diagnosed with toxoplasmosis. As toxoplasmosis is zoonotic, the remaining 68 pregnant sows present in the same room were culled 2 weeks after the outbreak of this disease.
Although clinical toxoplasmosis has been reported in young piglets, little was known of abortions associated with T. gondii and rates of congenital infection in pigs [2]. Epidemiologically, porcine toxoplasmosis has been classified into sporadic neonatal, postnatal, and epizootic infection [2]. Sporadic fatal toxoplasmosis in piglets has been reported in several countries, including the USA, Japan, and Korea. Although many infected piglets were born dead or sick, or became sick within 3 months of birth, others remained clinically normal.
Epizootic outbreaks of toxoplasmosis have been reported in Italy [4], Singapore [13], and Taiwan [18]. In Italy, there were 4 simultaneous outbreaks of toxoplasmosis with high mortality in different pig herds in 2 different provinces over a 1 month period [4]. The morbidity rate was as high as 60%, and the mortality rate ranged from 10% to 42% in fattening pigs weighing 60 to 180 kg. In Taiwan, 51 of 66 pregnant gilts infected with T. gondii on a single farm aborted within 2 months [18]. Most fetuses were stillborn, some were mummified, and few were born alive but died within a few days. In Singapore, a large outbreak of toxoplasmosis was observed in a herd containing 540 pigs [13]. Many pigs in different age groups, ranging from piglets to sows, became sick and died. The overall morbidity rate was 35.7% and the overall mortality rate was 11.8%.
In this study, higher abortion rates, up to 44%, were observed and unusually high sow mortality rates, up to 19%, were primarily associated with toxoplasmosis over a very short period of time. All clinical signs were restricted to pregnant sows, especially those at any stages of gestation. Although the sample size was relatively small, the high seroprevalence in aborted sows indicated an active toxoplasmosis infection in this particular herd. Transplacental infection of T. gondii were also observed. In sum, most clinical aspects and laboratory results in this herd were very similar to epizootic toxoplasmosis reported in other countries.
At the initial outbreak, toxoplasmosis was not suspected. Therefore, a thorough epidemiological survey was not conducted to determine the origin of T. gondii. In addition, the feed could not be investigated due to a disagreement with the farmer. All remaining pregnant sows were destroyed 2 weeks after the disease outbreak. Hence, the precise origin of T. gondii remains unknown. Studies aiming to clarify the sources of pig infection for T. gondii have suggested that ingestion of oocysts in contaminated feed, water, soil, and living animals were the main sources of infection [2,14]. In some countries, eating infected rodents has been regarded as a source of infection [16]. Cannibalism has been shown experimentally to be another possible route of infection [2]. However, most studies have suggested that oocysts shed by cats are the most common source. Cats may excrete millions of oocysts after ingesting only one bradyzoite or one tissue cyst, and many tissue cysts may be present in one infected mouse [3,6]. Although oocysts are shed only for a short period (1-2 weeks) in the life of a cat, the enormous numbers shed assure widespread contamination of the environment [6].
According to the system used to manage this herd, the pigs in individual rooms of the second pen were fed different feed. As they drank the same water from a private well and new animals had not been introduced into the herd in the period leading up to the disease outbreak, a change of feed source was the only variation within this herd. Because the disease outbreak in this herd was restricted to pregnant sows housed in the second room, and there was no recurrence of infection after the other pregnant sows were destroyed, the source of the parasite may have been the feed source or an animal contaminated with oocysts. Recently, the density of stray cats has been gradually increased in Jeju Island. Many wild rodents and stray cats were freely introduced into the old-fashioned small farms in Jeju. Although the feed for the sows were not able to be investigated, it is assumed that the feed, which had an abnormal stink, might have been contaminated in some way with feces contained T. gondii oocysts from wild animals, such as stray cats. According to a previous study, the seropositive rate of T. gondii in the residents of Jeju Island is relatively high compared to that of the past 30 years in Korea [20]. This suggested that incomplete cooked porcine meat and mammals such as pigs and deer may acts as reservoir hosts for T. gondii on Jeju Island. To prevent T. gondii infection in pigs, pig farms should be treated periodically with rodenticides, and no cats and wild rodents should be allowed to enter their living quarters. Moreover, animal feed should be carefully stored to prevent contamination by cats.
Figures and Tables
Acknowledgments
This work was supported by a grant (Code #20070401034009) from the BioGreen 21 Program run by the Rural Development Administration of Korea.
References
1. Cha SH, Choi EJ, Park JH, Yoon SR, Kwon JH, Yoon KJ, Song JY. Phylogenetic characterization of classical swine fever viruses isolated in Korea between 1988 and 2003. Virus Res. 2007. 126:256–261.

3. Dubey JP. Oocyst shedding by cats fed isolated bradyzoites and comparison of infectivity of bradyzoites of the VEG strain Toxoplasma gondii to cats and mice. J Parasitol. 2001. 87:215–219.

4. Gelmetti D, Sironi G, Finazzi M, Gelmini L, Rosignoli C, Cordioli P, Lavazza A. Diagnostic investigations of toxoplasmosis in four swine herds. J Vet Diagn Invest. 1999. 11:87–90.

5. Han DU, Lee CG, Kang MI, Jang H, Kim HS, Kim HJ, Wee SH. Serological studies on Toxoplasma gondii, hantavirus and some rickettsial pathogens in stray cats in Korea. Korean J Vet Public Health. 1999. 23:301–310.
6. Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002. 8:634–640.
7. Hill DE, Chirukandoth S, Dubey JP, Lunney JK, Gamble HR. Comparison of detection methods for Toxoplasma gondii in naturally and experimentally infected swine. Vet Parasitol. 2006. 141:9–17.

8. Hwang EK, Kim JH, Kim BH, Park CK, Choi SH. Infectious agents associated with swine abortions and stillbirths in Korea. RDA J Vet Sci. 1998. 40:48–53.
9. Kang HW, Kang SC, Yang HS, Bae JH, Kim JH. Coinfection of canine distemper virus and Toxoplasma gondii in a dog. J Vet Clin. 2004. 21:80–82.
10. Kim JH, Hwang EK, Kim YJ, Sohn HJ. Pathologic studies in piglets naturally infected with porcine reproductive and respiratory syndrome virus. Korean J Vet Pathol. 1997. 1:125–133.
11. Kim JH, Lee JK, Hwang EK, Kim DY. Prevalence of antibodies to Neospora caninum in Korean native beef cattle. J Vet Med Sci. 2002. 64:941–943.

12. Kim JH, Roh IS, Sohn HJ, Jean YH, Hwang EK, Yoon KJ. Porcine circovirus infection in weaned pigs with postweaning multisystemic wasting syndrome in Korea. Korean J Vet Res. 2003. 43:463–469.
13. Koh JGW, Loh H, Teng MF, Cheok WC. Toxoplasmosis in a pig herd. Singapore Vet J. 1978. 2:17–22.
14. Lehmann T, Graham DH, Dahl E, Sreekumar C, Launer F, Corn JL, Gamble HR, Dubey JP. Transmission dynamics of Toxoplasma gondii on a pig farm. Infect Genetic Evol. 2003. 3:135–141.
15. Lindsay DS, Blagburn BL, Dubey JP. Straw BE, D'Allaire S, Mengeling WL, Taylor DI, editors. Coccidia and other protozoa. Diseases of Swine. 1999. 8th ed. Ames: Iowa State University Press;661–664.

16. Lubroth JS, Dreesen DW, Ridenhour RA. The role of rodents and other wildlife in the epidemiology of swine toxoplasmosis. Prev Vet Med. 1983. 1:169–178.

17. Lyoo YS, Park CK, Chang CH. Diagnostic Manual for Animal Diseases. 1997. Seoul: LeeKong World;3–39.
18. Pan IC, Young SS, Wang CT, Yeh YC, Pan IJ, Chen HC. Toxoplasmosis in domestic animals: abortion and stillbirth in asymptomatic carrier gilts. Bull Inst Zool Acad Sin. 1962. 1:89–100.
19. Roh IS, Han JH, Kim JH, Ahn BW. Toxoplasmosis in piglets. Korean J Vet Res. 1997. 37:817–823.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download