Abstract
The effect of NaCl plus 3% chitosan on the systolic blood pressure of spontaneously hypertensive rats (SHR) were evaluated and compared with NaCl plus KCl (NaCl, 49.36% + KCl 49.36%) and chitosan or NaCl treatment alone. In SHR, administration of NaCl plus chitosan (44 mM Na/day) for two months significantly decreased the systolic blood pressure greater than of NaCl plus KCl and NaCl alone. NaCl plus chitosan resulted, though not statistically significant, in decreased urinary Na+ excretion and decreased blood urea nitrogen levels. Urinary creatinine of NaCl plus chitosan was slightly decreased compared to 3 treated groups. Serum electrolytes levels, however, remained unchanged. The combination of NaCl and chitosan may be superior to the conventional use of NaCl plus KCl or NaCl alone in the prevention of hypertension. Even though these supplementary diets have demonstrated potential anti-hypertensive effects in the experimental animal model, further research is needed before any recommendations can be made.
Hypertension is one of the most common cardiovascular diseases and has become a worldwide problem of epidemic proportions, affecting 15-20% of all adults [10,11,22,26]. It is the most serious common chronic health problem because it is a significant risk factor for the development of arteriosclerosis, stroke, myocardial infarction, and end-stage renal disease [25]. Chloride ions (Cl-) may play a role in the development and severity of age-related hypertension [23]. Therefore, decreasing the dietary intake of sodium chloride (NaCl) is generally recommended [23,25].
Hypertension is explained by the physiological and biochemical reactions of peripheral renin-angiotensin system, and its treatment is focused on the inhibition of angiotensin converting enzyme (ACE) activities for direct inhibition of hypertension. The naturally occurring polysaccharide, antihypertensive, biopolymer chitosan is an ideal candidate for an ACE inhibitor because it is considered to be milder and safer as compared with the drugs commonly used in the treatment of hypertension. In addition, chitosan is usually well-absorbed and demonstrates a multitude of other beneficial physiological properties [8,15].
The naturally occurring biopolymer, chitin-chitosan, is a well-known food supplement that effectively lowers blood cholesterol concentration and controls obesity [19]. In addition, it has many other useful biomedical applications-e.g., an absorbable suture, a drug carrier, an antitumor agent, a hemostatic agent, and a wound-healing agent. Chitosan itself has been developed as a new physiologically bioactive material, which has been touted as a treatment for various disorders, including asthma, atopic dermatitis, arteriosclerosis, hypertension, macular degeneration, arthritis, cancer, diabetes, and osteoporosis, among others [8,14,15].
This study was undertaken to examine the possible effects of chitosan alone and in combination with NaCl in spontaneously hypertensive rat (SHR) models. We also performed a comparative evaluation with the conventional use of NaCl plus KCl in an effort to find a better antihypertensive table salt for people to whom salt-restricted diets are indicated [13,17,18,27].
Chitosan was prepared by Biotech (Korea). To produce chitin, crab shells were ground into 180-250 mm particles with a ball mill. The powder was then placed in 2N HCl solution in room temperature for 12 h. Calcium, other inorganic materials, and proteins were then removed with 1 N NaOH to produce chitin. The chitin was then centrifuged and washed with distilled water, ethanol, and ether, in order, and vacuum dried at 70℃ to produce refined chitin in white powder form. It was then hydrolyzed with 50% NaOH solution at 100℃ for 5 h followed by 90% degree of deacetylation to produce chitosan containing 5% water and having a molecular weight of 100,000. A total of 52.5 g of flake-type chitosan was mixed with 950 g of 3% sodium acetate solution using a stirrer and filtered with nylon filter (200 mesh) to acquire a 5% chitosan solution (pH 4.5). A total of 324 g of natural sea salt (NaCl) containing 8% water, measured with a moisture meter (MA30-000v3; Sartorius AG, Germany), was dissolved in 1,000 ml of distilled water, followed by the addition of 180 ml of 5% chitosan solution. The resulting mixture was heated at 90℃ for required concentration by evaporation of water.
In vivo experiments were performed following the guidelines for the care and use of laboratory animals approved by Seoul National University [Approval No. SNU-070119-1]. Twenty-five SHR, 6 weeks of age and weighing 280-310 g (male) were purchased from Central Laboratory Animal, Korea. Animals were maintained in a certified animal house under supervision and standard conditions of 22 ± 2℃ and 55 ± 10% relative humidity with a photoperiod of 12 : 12 h of light : darkness. Water and a dry pellet diet (Purina Rodent Laboratory Chow; Ralston Purina, USA) were given ad libitum. The rats were acclimatized for 4 days prior to the start of the experiments and randomly allocated to five groups. Group 1: NaCl + KCl (49.36% NaCl plus 49.36% KCl), group 2: NaCl + chitosan (NaCl plus 3% chitosan), group 3: NaCl, group 4: chitosan (3%) administered orally using a metal gastric zonde, and group 5; untreated control. The concentration of sodium given was 44 mM (1 g of sodium) / day [3].
After two months of consuming their respective diets, all rats were anaesthetized by an intramuscular injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) into the right quadriceps femoris muscle. Blood and urine were collected from the heart and urinary bladder, respectively, followed by cervical dislocation.
The body weights of the rats were measured once per week at the same time during the day. Measurement of the systolic blood pressure was performed once per week at the same time during the day. After the stabilization of the animals in a warm box at 37℃ for 15 min, the tail systolic blood pressure was measured with a non-invasive blood pressure system (ML125/R; AD Instruments Power Lab System, USA) and was reported as the mean value of three consecutive measurements [28].
Blood was centrifuged at 3,000 g for 15 min to obtain serum. After serum separation, we measured the levels of electrolytes [sodium (Na+), chlorine (Cl-) and potassium (K+)] by an electrolyte measurement apparatus based on an ion electrode method. Angiotensin I and II were measured with a rat angiotensin I and II EIA kits (Phoenix Pharmaceuticals, USA) according to the manufacturer's instructions.
Urine was assayed for creatinine by a refractometer (Atago, Japan), blood urea nitrogen (BUN) by a commercial kit (BUN Kainos; Kainos, Japan) according to a modified urease-indole-phenol method and electrolytes (Na+, Cl- and K+) by an electrolyte measurement apparatus based on an ion electrode method [6].
The autopsied heart and kidneys from five rats in each dietary group of SHR were fixed in 10% formalin buffer for 48 h, followed by dehydration in an alcohol-xylene series prior to embedding in paraffin wax. The glomerular, vascular, tubular, and interstitial changes were graded from 0 to 3 observing H&E stained slides (0 = normal; 0.5 = minimal; 1 = slight; 2 = moderate and 3 = severe) [1].
None of the animals died. All groups had an increase in body weight during the experimental period. At the end of the experiment, the control group gained 74.34 ± 10.91 g from baseline, while the chitosan, chitosan plus NaCl, and KCl plus NaCl treated groups increased by 77.67 ± 8.70 g, 74.16 ±10.40 g, and 61.53 ± 14.70 g, respectively. Throughout the experimental period, no statistically significant difference in body weight changes was observed between all treated groups and control group (Fig. 1). The food intake was essentially proportional to its change in weight (data not shown).
A continuous increase (control: 195.60 ± 7.90 to 215.50 ± 5.20 mmHg) in the systolic blood pressure (SBP) was seen in all the groups during the experimental period. In general, SBP of the NaCl plus chitosan-treated group was lower than that of the KCl plus NaCl-treated group (232.50 ± 7.60 mmHg) and the NaCl-treated group and higher than that of the chitosan-treated group (212.40 ± 5.70 mmHg) and control group. There was a significant decrease (p < 0.05) in SBP in the pure NaCl plus chitosan group at 2 week only when compared to the KCl plus NaCl treated group, but not at 8th week (Fig. 2).
Angiotensin I and II concentration, NaCl plus chitosan diet showed 4.71 ± 1.50 ng/ml which was 4.89% higher angiotensin I than control diet (4.49 ± 0.88 ng/ml). KCl plus NaCl diet showed 10.46% less angiotensin I than the control. Angiotensin II of NaCl plus chitosan and KCl plus NaCl was decreased to 2.44% and 0. 85%, respectively, compared to the control group. No consistent differences in final serum angiotensin I and II were seen among the five groups (Fig. 3).
In this study, serum electrolytes were similar and unchanged in all groups (Fig. 4). In general, sodium levels were the highest followed by chlorine and potassium. Na+, K+ and Cl- levels in the urine did not differ significantly between the control and the test groups regardless of treatments, but there was a tendency toward an decrease in urinary Na+ excretion when treated with NaCl plus chitosan compared to NaCl + KCl or NaCl alone group (Fig. 5).
The NaCl plus chitosan treated group had decreased BUN levels compared to NaCl + KCl or NaCl alone groups, though none of these were statistically significant (p < 0.05).
BUN levels were the lowest (2564.00 ± 454.37 mg/ dl) in the NaCl plus chitosan treated group and highest (3006.00 ± 1236.82 mg/dl) in the control group, followed by the NaCl plus KCl groups (2838.80 ± 858.77 mg/dl) (Fig. 6A).
Levels of urinary creatinine (Fig. 6B) significantly decreased in all four treated groups compared to the control (133.96 ± 51.37 mg/dl). The creatinine was lowest in the NaCl plus chitosan group (80.06 ± 22.98 mg/dl); therefore, the proximal tubules were thought to be less disturbed in the rats exposed to daily levels of NaCl plus chitosan over a period of 8 weeks.
Levels of urinary creatinine (Fig. 6B) significantly decreased in all four treated groups compared to the control (133.96 ± 51.37 mg/dl) (p < 0.05). The creatinine was lowest in the NaCl plus chitosan group (80.06 ± 22.98 mg/ dl); therefore, the proximal tubules were thought to be less disturbed in the rats exposed to daily levels of NaCl plus chitosan over a period of 8 weeks.
Hypertension is a major risk factor for cardiovascular diseases such as heart failure, stroke, coronary artery disease, and myocardial infarction [12]. It is called the 'silent killer' for good reason: Almost one-third of individuals with hypertension do not know that they have it and almost 50% of those who do know they have hypertension do not control it properly. Hypertension is the primary or a contributing cause of death in over 200,000 patients per year in the United States alone [20]. Therefore, there is an urgent need for significant research to develop new medicine to treat hypertension. There are a great number of pharmaceuticals that have been proven to be effective in lowering blood pressure, but usually have side effects [5]. Diet and lifestyle modification may also be effective tools for the prevention of hypertension, which could decrease the need for antihypertensive drugs [2].
The link between sodium intake and hypertension remains controversial due to inconsistencies between early epidemiologic studies, which showed a strong positive relationship between salt intake, blood pressure, and the incidence of hypertension, and more recent studies, which showed only modest decreases in blood pressure with sodium reduction, particularly in the normotensive population [3]. Chrysant et al. [3] reported an increased risk of heart attacks and cardiovascular mortality in persons who appeared to have restricted their sodium intake, suggesting that sodium reduction may be harmful under some circumstances. In this respect, the search for diet- related preventive measures against hypertension is obviously of interest and within the scope of functional foods.
In the present study, we compared the consumption of an effective amount of NaCl plus KCl, NaCl, NaCl plus chitosan, and chitosan by SHRs in an effort to find a suitable agent for salting food that has saltiness of NaCl, but with antihypertensive effects. We were particularly interested in the SHR rats because they represent an animal model of the genetic predisposition to develop arterial hypertension during aging, and they have numerous similarities to humans with essential hypertension [2,21].
This study showed that body weight increased progressively with aging and in all groups to a similar extent. The control SHR gained more weight than the other groups, and after week 8, the body weight tended to be lower in the NaCl plus chitosan group than that in the chitosan alone group, but not to a significant extent. NaCl plus 3% chitosan tended to reduce the blood pressure in SHRs with greater efficacy than NaCl plus KCl and NaCl alone.
Urinary electrolyte concentrations of Cl- increased only when KCl was supplemented with NaCl and the level of Na+, K+ and Cl- was lower when SHRs were treated with NaCl plus chitosan compared to NaCl alone. The serum electrolyte concentrations of Na+ and K+ were identical across all groups. On the other hand, the BUN levels of the NaCl plus chitosan-treated groups were lower than that of the control group. This finding on the BUN level suggested a decrease in glomerular filtration which may be explained by the decrease of blood pressure due to the increase of angiotensin I or the decrease of angiotensin II production [7,9,16,24]. Since urea is a final product in protein metabolism and is excreted in the urine via the kidney, the BUN level is important in the evaluation of renal function. Although urinary creatinine unexpectedly increased in control group, it was the lowest in the NaCl plus chitosan group. This finding indicates that the anti-hypertensive effect of NaCl plus chitosan may be due to the amelioration of kidney function in the experimental animal models. This combination may be a better option for dietary supplementation than the conventional uses of NaCl plus KCl or NaCl alone.
This study concluded that the consumption of NaCl plus chitosan - based functional dietary salt should be encouraged as part of an overall lifestyle medicine approach for the prevention of hypertension. To our knowledge, this is the first report showing the antihypertensive effect of a composition of NaCl plus chitosan. This composition may be applied as a substitute table salt for imparting saltiness to dishes or as an ingredient in crackers, snack foods, and other food products requiring salt, which would be particularly appropriate for patients recommended to decrease the amount of salt in their diet. As there are several limitations in this study, further research is needed to identify bioactive compound(s) and the anti-hypertensive mechanism(s) of action of NaCl plus chitosan.
Figures and Tables
 | Fig. 1Changes in body weight of spontaneously hypertensive rats administered dietary salts over the experimental period. Vertical bars represent the mean ± SD (n = 5). |
 | Fig. 2Changes in systolic blood pressure of spontaneously hypertensive rats administered various combinations of dietary salts. Means with the same alphabetical letter are not significantly different (p < 0.05). Vertical bars represent the mean ± SD (n = 5). |
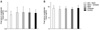 | Fig. 3Effect of dietary salts on serum angiotensin 1 and 2 concentrations (3A and 3B). Vertical bars represent the mean ± SD (n = 5). |
 | Fig. 4Effect of dietary salts on serum electrolytes (Na+, K+ and Cl-). Vertical bars represent the mean ± SD (n = 5). |
Acknowledgments
This work was supported by grants provided by the Korea Research Foundation (KRF-2006-J02901) and BK 21 project, Korea. Dr. N. K. Dutta was supported by a fellowship from the Korea Research Foundation.
References
1. Boorman GA, Eustis SL, Elwell MR, Montgomery CA. Jr., MacKenzie WF. Pathology of the Fischer Rat. 1990. San Diego: Academic Press;132–137. 146–151.
2. Chen Q, Xuan G, Fu M, He G, Wang W, Zhang H, Ruan H. Effect of angiotensin I-converting enzyme inhibitory peptide from rice dregs protein on antihypertensive activity in spontaneously hypertensive rats. Asia Pac J Clin Nutr. 2007. 16:Suppl 1. 281–285.
3. Chrysant GS, Bakir S, Oparil S. Dietary salt reduction in hypertension--what is the evidence and why is it still controversial? Prog Cardiovasc Dis. 1999. 42:23–38.

4. de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int. 2004. 66:2454–2466.

5. Deshmukh M, Lee HW, McFarlane SI, Whaley-Connell A. Antihypertensive medications and their effects on lipid metabolism. Curr Diab Rep. 2008. 8:214–220.

6. Fukuda S, Tsuchikura S, Iida H. Age-related changes in blood pressure, hematological values, concentrations of serum biochemical constituents and weights of organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp Anim. 2004. 53:67–72.

7. Hutchinson JS, Mendelsohn FA, Doyle AE. Blood pressure responses of conscious normotensive and spontaneously hypertensive rats to intracerebroventricular and peripheral administration of captopril. Hypertension. 1980. 2:546–550.

8. Je JY, Park PJ, Kim B, Kim SK. Antihypertensive activity of chitin derivatives. Biopolymers. 2006. 83:250–254.

9. Je JY, Park JY, Jung WK, Park PJ, Kim SK. Isolation of angiotensin I converting enzyme (ACE) inhibitor from fermented oyster sauce, Crassostrea gigas. Food Chem. 2005. 90:809–814.

10. Jenei Z, Páll D, Katona E, Kakuk G, Polgár P. The epidemiology of hypertension and its associated risk factors in the city of Debrecen, Hungary. Public Health. 2002. 116:138–144.

11. Jo I, Ahn Y, Lee J, Shin KR, Lee HK, Shin C. Prevalence, awareness, treatment, control and risk factors of hypertension in Korea: the Ansan study. J Hypertens. 2001. 19:1523–1532.

12. Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996. 275:1571–1576.

13. Kim D, Yokozawa T, Hattori M, Kadota S, Namba T. Effects of aqueous extracts of Apocynum venetum leaves on spontaneously hypertensive, renal hypertensive and NaCl-fed-hypertensive rats. J Ethnopharmacol. 2000. 72:53–59.

14. Kimura Y, Okuda H. Prevention by chitosan of myelotoxicity, gastrointestinal toxicity and immunocompetent organic toxicity induced by 5-fluorouracil without loss of antitumor activity in mice. Jpn J Cancer Res. 1999. 90:765–774.

16. Koike H, Ito K, Miyamoto M, Nishino H. Effects of long-term blockade of angiotensin converting enzyme with captopril (SQ14,225) on hemodynamics and circulating blood volume in SHR. Hypertension. 1980. 2:299–303.

17. Majima M, Hayashi I, Inamura N, Fujita T, Ogino M. A nonpeptide mimic of bradykinin blunts the development of hypertension in young spontaneously hypertensive rats. Hypertension. 2000. 35:437–442.

18. Mélançon S, Bachelard H, Badeau M, Bourgoin F, Pitre M, Larivière R, Nadeau A. Effects of high-sucrose feeding on insulin resistance and hemodynamic responses to insulin in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2006. 290:H2571–H2581.

19. Mhurchu CN, Dunshea-Mooij C, Bennett D, Rodgers A. Effect of chitosan on weight loss in overweight and obese individuals: a systematic review of randomized controlled trials. Obes Rev. 2005. 6:35–42.

20. Muntner P, Krousel-Wood M, Hyre AD, Stanley E, Cushman WC, Cutler JA, Piller LB, Goforth GA, Whelton PK. Antihypertensive prescriptions for newly treated patients before and after the main antihypertensive and lipid-lowering treatment to prevent heart attack trial results and seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure guidelines. Hypertension. 2009. 53:617–623.

21. Nakamura S, Averill DB, Chappell MC, Diz DI, Brosnihan KB, Ferrario CM. Angiotensin receptors contribute to blood pressure homeostasis in salt-depleted SHR. Am J Physiol Regul Integr Comp Physiol. 2003. 284:R164–R173.
22. Singh RB, Suh IL, Singh VP, Chaithiraphan S, Laothavorn P, Sy RG, Babilonia NA, Rahman AR, Sheikh S, Tomlinson B, Sarraf-Zadigan N. Hypertension and stroke in Asia: prevalence, control and strategies in developing countries for prevention. J Hum Hypertens. 2000. 14:749–763.

23. Tanaka M, Schmidlin O, Yi SL, Bollen AW, Morris RC Jr. Genetically determined chloride-sensitive hypertension and stroke. Proc Natl Acad Sci USA. 1997. 94:14748–14752.
24. Terragno DA, Crowshaw K, Terragno NA, McGiff JC. Prostaglandin synthesis by bovine mesenteric arteries and veins. Circ Res. 1975. 36:Suppl 1. 76–80.

25. Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, Thom S McG. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004. 18:139–185.

26. Wu DM, Pai L, Chu NF, Sung PK, Lee MS, Tsai JT, Hsu LL, Lee MC, Sun CA. Prevalence and clustering of cardiovascular risk factors among healthy adults in a Chinese population: the MJ Health Screening Center Study in Taiwan. Int J Obes Relat Metab Disord. 2001. 25:1189–1195.

27. Yang HY, Yang SC, Chen JR, Tzeng YH, Han BC. Soyabean protein hydrolysate prevents the development of hypertension in spontaneously hypertensive rats. Br J Nutr. 2004. 92:507–512.

28. Zhu YZ, Wang ZJ, Zhu YC, Zhang L, Oakley RM, Chung CW, Lim KW, Lee HS, Ozoux ML, Linz W, Böhm M, Kostenis E. Urotensin II causes fatal circulatory collapse in anesthesized monkeys in vivo: a "vasoconstrictor" with a unique hemodynamic profile. Am J Physiol Heart Circ Physiol. 2004. 286:H830–H836.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download