Abstract
Primary testicular tumors are the most common causes of cancer in male dogs. Overall, the majority of canine patients should be cured by testicular surgery. However, tumor markers are not well-known in veterinary medicine. We sought to determine using immunohistochemistry whether the combined human testicular tumor markers (placental alkaline phosphatase, OCT3/4, CD30, alpha-fetoprotein, inhibin-alpha, vimentin, c-KIT, and desmin) are expressed in canine seminomas and Sertoli cell tumors (SCTs). We examined 35 canine testicular tumors, 20 seminomas and 15 SCTs. c-KIT was expressed markedly in canine seminomas. Both inhibin-alpha and vimentin were expressed significantly in canine SCTs. The results of this study demonstrate differences and similarities between tumor marker expression of testicular tumors in dogs and humans. All the main markers in current routine use are discussed as well as potential useful markers for benign and malignant tumors, and tumor progression.
Testicular tumors arise from germ cells and sex-cord stromal elements of the testis [22], and are divided into four general categories: germ cell tumors including seminoma, teratoma, embryonal carcinoma, and yolk sac carcinoma arising from the germinal epithelium of the seminiferous tubules; sex-cord stromal tumors, including Sertoli cell tumor (SCT) and Leydig (interstitial) cell tumor; mixed germ cell sex-cord stromal tumors; and primary tumors not specific to the testis [22,30,32]. The major tumors are seminoma and SCT, which occur with equal frequency, showing a prevalence of 0.068-4.6% in mature and old male dogs [30,31,34]. Seminoma is derived from the germ cells that constitute the spermatogenic epithelium within the seminiferous tubules. SCT arises from the supporting cells within the seminiferous tubules. It is most common in dogs, but has also been reported in stallions, rams, bulls, goats, and cats [21,23].
In humans, testicular tumors are the most common cancers found in men between 15-35-years-of-age, where 90% are germ cell tumors [14,26,32]. Since the rate of testicular neoplasms is increasing, much research has been carried out to more accurately diagnose and manage these patients [2,11]. In particular, many immunohistochemical markers have been introduced to accurately establish histological diagnoses and to investigate tumor pathogenesis [3,18]. Markers including cytokeratins, c-KIT, CD30, epithelial membrane antigen, inhibin-alpha, OCT3/4, placental alkaline phosphatase (PLAP) and alpha fetoprotein (AFP) are sensitive and specific for the diagnosis of human testicular tumors [13,17,27,35]. Although tumor markers in canine testicular tumors have been studied, relatively less is known, which presently limits their use in diagnosis and evaluation of tumor pathogenesis.
In the present study, we examined tumor markers including PLAP, AFP, inhibin-alpha, vimentin, OCT3/4, CD30, desmin, and c-KIT to determine expression of these proteins and an appropriate antibody panel. To accomplish this, we employed immunohistochemical staining of canine seminomas and SCTs.
Testicular specimens from 35 male dogs with seminomas (n = 20) or SCTs (n = 15) were obtained from the files of the Department of Pathobiology, Small Animal Tumor Diagnostic Center, Konkuk University, Seoul, Korea. In addition, two normal testicular samples from neutered male dogs were used as negative controls. Histopathological analyses based on hematoxylin and eosin staining were performed.
Each testis was fixed in 10% neutral buffered formalin. Blocks that contained testis tumor tissue were embedded in paraffin wax. Serial 4 µm-thick sections were acquired from each paraffin-block for immunohistochemical staining. Monoclonal and polyclonal antibodies (Table 1) included PLAP (Biogenex, USA), AFP (Biogenex), inhibin-alpha (Dako, USA), vimentin (Dako), OCT3/4 (Santa Cruz Biotechnology, USA), CD30 (Santa Cruz Biotechnology), desmin (Biogenex, USA), and c-KIT (Dako, USA). Sections were deparaffinized in xylene, rehydrated in a graded ethanol series, treated with 3% hydrogen peroxide solution for 20 min at room temperature, and washed three times with phosphate-buffered saline (PBS; pH 7.4, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4). For inhibin-alpha, OCT3/4 and CD30, antigen retrieval was performed by heating slides in Tris-EDTA buffer (pH 9) in a microwave oven (650 W, high power) for 20 min. After 3 min washes with PBS, sections to be stained with AFP, vimentin, and c-KIT antibodies were incubated in a blocking solution of 5% normal goat serum (Vector Laboratories, USA) for 30 min. All primary antibodies were incubated with sections overnight at 4℃. To "visualize" immunolabeling, a two-step EnVision system (Dako, USA) was applied after removal of the primary antibody. In this system, EnVision rabbit/mouse reagent conjugated to horseradish peroxidase was applied for 40 min at room temperature. The slides were subsequently washed four times in PBS and incubated with the supplied substrates until the desired color intensity developed. The reaction was stopped by washing in distilled water. Sections were counterstained with Harris hematoxylin.
Images were acquired using an Olympus BX41 microscope (Olympus, USA) fitted with a Leica DFC 290 digital camera (Leica, Switzerland) and analyzed for positive signals using Image-Pro Plus software ver. 4.1 (Media Cybernetics, USA). The immunolabeling was scored as positive if > 10% of tumor cells displayed staining.
The results of immunohistochemical studies are summarized in Tables 2 and 3. PLAP, AFP, inhibin-alpha, vimentin, desmin, and c-KIT were expressed in seminomas and SCTs, while OCT3/4 and CD30 were not expressed. In normal Leydig cells, inhibin alpha, vimentin, and desmin antibodies were weakly immunoreactive. Normal Sertoli cells had focal staining for vimentin and c-KIT. PLAP, AFP, OCT3/4, and CD30 produced no immunoreactivity in normal testis (data not shown).
Seminomas consisted of aggregates of germ cells that filled the lumens or sheets of the affected seminiferous tubules. These cells replaced the normal lining of spermatogenic and Sertoli cells. Large and irregular cellular aggregates and small clusters of cells were seen to varying extents. Dense fibrous bands subdivided the tumor into large discrete nodules. The tumor cells were large and polyhedral with vesicular nuclei and prominent nucleoli (Fig. 1A). PLAP staining was moderate-to-diffuse in four cases. AFP and inhibin-alpha were positive in eight and nine cases, respectively. AFP was diffusely expressed in tumor cells (Fig. 1B), while inhibin-alpha demonstrated diffuse immunoreactivity (Fig. 1C). Vimentin and desmin were positive in five and six separate cases, respectively. Expression of vimentin produced strongly positive immunoreactivity in some tumor cells within some seminomas (Fig. 1D). Expression of desmin was diffuse and focal to the cytoplasm of tumor cells (Fig. 1E). No OCT3/4 or CD30 staining was evident in the tumors (data not shown). C-KIT was strongly positive in all cases (Fig. 1F).
In SCTs, there was abundant fibrous tissue stroma. Cells within the tumor resembled Sertoli cells that normally populate the seminiferous tubules, and were arranged into sheets or tubules separated by fibrous connective tissue. The tumor cells had small, round or elongated nuclei with an eosinophilic cytoplasm (Fig. 2A). Of 15 SCT samples, six cases were positive for PLAP with weak and focal immunostaining. In eight cases, AFP was expressed in the cytoplasm of tumor cells (Fig. 2B). Fourteen cases were positive for inhibin-alpha and vimentin. While the tumor cells were strongly positive for inhibin-alpha (Fig. 2C), they were only weakly positive for vimentin (Fig. 2D). All SCTs were negative for OCT3/4 (data not shown). CD30 was not expressed in tumor cells (data not shown). Focal immunostaining of desmin was observed in five cases (Fig. 2E). Ten cases were positive for c-KIT, where its expression was distributed diffusely but strongly in the cytoplasm of tumor cells (Fig. 2F).
Primary testicular tumors are common in mature and old male dogs [34]. The main types of testicular tumors in dogs are seminomas and SCTs, which are associated with chryptorchidism. In humans, testicular tumors are the most common cancers found in men, with an increasing rate of incidence [26,32]. Substantial research on human testicular tumors has resulted in more accurate diagnoses and better management of patients [3,11]. In particular, many tumor markers have been found for tumor pathogenesis [1,4,6]. Of these markers, cytokeratins, c-KIT, CD30, epithelial membrane antigen, inhibin-alpha, OCT3/4, PLAP, and AFP are widely used because of their sensitivity and specificity. The usefulness of these as tumor markers has been assessed in many studies [13,17,27,35]. Canine testicular tumor markers have been studied [23,28,34]. However, such veterinary tumor markers in male canine patients still require more study to be useful in accurate diagnosis. Thus, the purpose of our present study was to establish the specificity of tumor markers including PLAP, AFP, inhibin-alpha, vimentin, OCT3/4, CD30, desmin, and c-KIT in canine seminoma and SCT.
In the current seminoma series, c-KIT was found to be the most sensitive marker, because tumor cells of all seminoma cases were homogeneously and strongly positive for the antibody. c-KIT is a product of the c-KIT oncogene, which encodes a type III transmembrane tyrosine kinase receptor that is required in normal spermatogenesis. High expression of c-KIT is found in human testicular germ cell tumors, especially in human seminoma [26]. KIT signal transduction appears to be an important pathway for carcinogenesis of seminoma [12]. Our result demonstrates that c-KIT is potently expressed and is potentially a specific tumor marker in canine seminoma, similar to human tumors. OCT3/4, also known as otf3 or pou5f1, is a member of the POU family of transcription factors expressed in pluripotent mouse and human embryonic stem and germ cells [7,10,29]. Unexpectedly, OCT3/4, which is regarded as a highly sensitive marker for human seminoma and embryonal carcinoma [2,33], was not expressed in any canine seminoma. OCT3/4 may not be a reliably specific tumor marker in canine seminoma. PLAP, a membrane-bound enzyme normally synthesized by placental syncytiotrophoblasts [4], has been widely applied in the significant markers of human seminoma. In contrast, PLAP staining was detected weakly and heterogeneously in canine SCTs. Although a positive reaction was observed in 20% of cases, the result was less significant than that observed in human seminoma PLAP immunostaining. This result might be related with the classification of testicular tumors including two types of seminoma. The first type is classical seminoma (SE) and the second type is spermatocytic seminoma (SS). Grieco et al. [8] showed that neoplastic cells are immunoreactive for PLAP in all cases with SE. Neoplastic cells in SS are essentially negative for PLAP [8]. Therefore, our result combined with the observations of Grieco et al. [8] indicate that 20% cases displaying a positive reaction were likely SE and the 80% cases displaying negative reaction were SS. Inhibin-alpha, a subunit of inhibin, is secreted mainly from testicular Sertoli cells with an additional small contribution from Leydig cells [4,34]. Although little is known about the cells secreting inhibin in primary testicular tumors of humans and older animals [5,9,16], inhibin immunoreactivity has been biochemically estimated in human and canine testicular tumors. In the current seminoma series, inhibin-alpha was observed in the cytoplasm of tumors cell in 45% of the cases. Vimentin and desmin was expressed in 25% and 30%, respectively, of canine seminoma samples. As for vimentin and desmin, unlike the diffuse cytoplasmic staining present in SCTs, immunoreactivity in seminomas was observed confined to the cytoplasm of the tumor cells. Vimentin and desmin are known as markers of mesenchymal origin tumors including connective tissue, endothelium, hematopoietic cell, and muscle. Because of the origin of seminoma is germ cells originating from epithelial tissue, vimentin and desmin are not appropriate markers. CD30 is a membrane glycoprotein of the tumor necrosis factor receptor superfamily, which plays a useful role in identifying primary embryonal carcinoma in humans [33]. CD30 is also useful for distinguishing embryonal carcinoma from seminoma [19]. When the fixation is not adequate, seminoma may be confused with the solid pattern of embryonal carcinoma [36]. The distinction between seminoma and embryonal carcinoma is very important because the treatment differs. Our result shows that CD30 was not expressed in canine seminoma. Although there was no case of embryonal carcinoma in this study, this result suggests that CD30 can be used to differentiate embryonal carcinoma from seminoma.
These immunohistochemical results demonstrate that c-KIT is a sensitive marker for seminoma in dogs. In human seminoma, c-KIT is expressed in seminomas at the high prevalence rate of 88-100%, making it one of the most effective immunohistochemical markers [1,18,26]. Although PLAP, AFP, inhibin-alpha, desmin, and vimentin immunoreactivity were observed in this study, they did not demonstrate significant results.
The immunoreactivity of SCTs indicates that inhibin-alpha and vimentin were the most remarkable immunoreactivity of all the antibodies used in this study. Inhibin-alpha and vimentin were expressed with a high prevalence rate of 93.3% and were diffusely and moderately evident in the cytoplasm of neoplastic cells. Previous studies did not detect immunohistochemical expression of inhibin-alpha in SCTs in dogs [31,34]. In this study, however, this expression was observed in 93.3% of SCTs. Grootenhuis et al. [9] showed that peripheral levels of immunodetectable inhibin in dogs with SCTs are higher than those in normal dogs. Kawakami et al. [15] also demonstrated that blood plasma inhibin alpha concentration of dogs with SCT is higher than normal testis. In human testicular SCT, Iczkowski et al. [11] reported positive inhibin-alpha immunostaining in 10 of 11 (91%) testicular SCT. The results of our study combined with those of Grootenhuis et al. [9], Kawakami et al. [15] and Iczkowski et al. [11] show that inhibin-alpha is likely to be the material secreted from the neoplastic cells of SCTs. In the current series, inhibin alpha immunostaining of Sertoli cells was evident in normal testis. The staining intensity in normal Sertoli cells was consistently lower than in the tumor cells of SCTs. Sertoli cells are the main source of inhibin production in the male. This result also suggests that neoplastic Sertoli cells in SCTs produce abundant inhibin alpha. Vimentin is a major intermediate filament present in the cytoplasm of Sertoli cells, and it has been used to identify these cells [24]. In humans, vimentin immunostaining is positive in SCTs [20]. Similar to human studies, our canine study showed that vimentin might be still the intermediate filament forming the basic structure of the cells of canine SCTs. PLAP was positive in some cases, although, in theory, they should not be expressed in SCTs. In humans PLAP immunostaining is also negative in SCTs [16,20]. To more precisely determine the expression profile of these proteins in canine SCTs, further longitudinal studies should be performed. CD30 was not found in SCTs, which is consistent with the idea that CD30 is expressed only in embryonal carcinoma. OCT3/4 was not identified in SCTs. SCT and seminoma reportedly became OCT3/4-positive in humans, but such an immunoreaction in neoplastic cells was not presently demonstrated. Desmin was expressed focally, and only five cases were positive for the cytoplasm of tumor cells. Although c-KIT and AFP were expressed with a diffuse pattern in 10 cases of SCT, they were a limited marker compared with inhibin-alpha or vimentin.
Based on these results, c-KIT is the most sensitive marker in canine seminomas, while inhibin-alpha and vimentin are the most sensitive markers in canine SCTs. Although OCT3/4 and PLAP are regarded as the most suitable immunohistochemical markers in human germ cell tumors including seminoma, these were either not expressed or only weakly expressed in thecanine seminomas presently examined. One interpretation of these results might be that all the antibodies used in this experiment were produced against human proteins. As a result, these antibodies might fail to detect canine PLAP or OCT3/4. However, it is also reasonable to think that OCT3/4 and PLAP are not effective markers for canine seminoma. Inhibin-alpha and vimentin were expressed markedly in canine SCTs in a similar fashion as in human cells. Therefore, these proteins are specific markers in canine SCTs.
In conclusion, the results of this study demonstrate that there are differences and similarities between the expression of testicular tumor markers in dogs and humans. Further investigation is required to determine whether expression of c-KIT, inhibin alpha, vimentin, AFP, and PLAP is related with tumor metastasis or malignancy, and to elucidate the role of these proteins in the development of canine testicular tumors.
Figures and Tables
Fig. 1
Immunohistochemical markers in canine seminoma. (A) Seminomas consisted of aggregates of germ cells that filled the affected seminiferous tubules. The tumor cells were large and polyhedral with vesicular nuclei and prominent nucleoli. H&E stain. (B-F) Positive signals to tumor cells; (B) alpha-fetoprotein, (C) inhibin-alpha, (D) vimentin, (E) desmin and (F) c-KIT. Immunostain and counterstain with Harris hematoxylin. Scale bars = A: 350 µm, B-F: 140 µm.
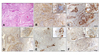
Fig. 2
Immunohistochemical markers in canine Sertoli cell tumors. (A) Cells within the tumor resemble Sertoli cells that normally populate the seminiferous tubules and are arranged into sheets or tubules separated by fibrous connective tissues. H&E stain. (B-F) Positive signals to tumor cells; (B) alpha-fetoprotein, (C) inhibin-alpha, (D) vimentin, (E) desmin and (F) c-KIT. Immunostain and counterstain with Harris hematoxylin. Scale bars = A: 350 µm, B-F: 140 µm.

Acknowledgments
We thank Ms. R-H Jang for her excellent technical assistance. This study was supported in part by a grant for scientific animal research from the Ministry of Agriculture and Forestry of Korea.
References
1. Biermann K, Klingmüller D, Koch A, Pietsch T, Schorle H, Büttner R, Zhou H. Diagnostic value of markers M2A, OCT3/4, AP-2gamma, PLAP and c-KIT in the detection of extragonadal seminomas. Histopathology. 2006. 49:290–297.

2. Cheng L. Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer. 2004. 101:2006–2010.

3. Cheng L, Thomas A, Roth LM, Zheng W, Michael H, Karim FW. OCT4: a novel biomarker for dysgerminoma of the ovary. Am J Surg Pathol. 2004. 28:1341–1346.
4. Debora J, Junqi Q, David GB. Dabbs DJ, editor. Immunohistology of the prostate, bladder, testis and kidney. Diagnostic Immunohistochemistry. 2002. 2nd ed. New York: Churchill Livingstone;75–86.
5. De Jong FH, Grootenhuis AJ, Steenbergen J, van Sluijs FJ, Foekens JA, ten Kate FJ, Oosterhuis JW, Lamberts SW, Klijn JG. Inhibin immunoreactivity in gonadal and non-gonadal tumors. J Steroid Biochem Mol Biol. 1990. 37:863–866.

6. De Vico G, Papparella S, Di Guardo G. Number and size of silver-stained nucleoli (Ag-NOR clusters) in canine seminomas: correlation with histological features and tumour behaviour. J Comp Pathol. 1994. 110:267–273.

7. Goto T, Adjaye J, Rodeck CH, Monk M. Identification of genes expressed in human primordial germ cells at the time of entry of the female germ line into meiosis. Mol Hum Reprod. 1999. 5:851–860.

8. Grieco V, Riccardi E, Rondena M, Ciampi V, Finazzi M. Classical and spermatocytic seminoma in the dog: histochemical and immunohistochemical findings. J Comp Pathol. 2007. 137:41–46.

9. Grootenhuis AJ, van Sluijs FJ, Klaij IA, Steenbergen J, Timmerman MA, Bevers MM, Dieleman SJ, de Jong FH. Inhibin, gonadotrophins and sex steroids in dogs with Sertoli cell tumours. J Endocrinol. 1990. 127:235–242.

10. Hansis C, Grifo JA, Krey LC. Oct-4 expression in inner cell mass and trophectoderm of human blastocysts. Mol Hum Reprod. 2000. 6:999–1004.

11. Iczkowski KA, Butler SL. New immunohistochemical markers in testicular tumors. Anal Quant Cytol Histol. 2006. 28:181–187.
12. Izquierdo MA, Van der Valk P, Van Ark-Otte J, Rubio G, Germa-Lluch JR, Ueda R, Scheper RJ, Takahashi T, Giaccone G. Differential expression of the c-kit proto-oncogene in germ cell tumours. J Pathol. 1995. 177:253–258.

13. Jacobsen GK, Jacobsen M. Alpha-fetoprotein (AFP) and human chorionic gonadotropin (HCG) in testicular germ cell tumours. A prospective immunohistochemical study. Acta Pathol Microbiol Immunol Scand [A]. 1983. 91:165–176.
14. Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol. 2004. 28:935–940.
15. Kawakami E, Hirano T, Hori T, Tsutsui T. Testicular superoxide dismutase activity, heat shock protein 70 concentration and blood plasma inhibin-alpha concentration of dogs with a Sertoli cell tumor in a unilateral cryptorchid testis. J Vet Med Sci. 2007. 69:1259–1262.

16. Kommoss F, Oliva E, Bittinger F, Kirkpatrick CJ, Amin MB, Bhan AK, Young RH, Scully RE. Inhibin-alpha CD99, HEA125, PLAP, and chromogranin immunoreactivity in testicular neoplasms and the androgen insensitivity syndrome. Hum Pathol. 2000. 31:1055–1061.

17. Koshida K, Uchibayashi T, Yamamoto H, Hirano K. Significance of placental alkaline phosphatase (PLAP) in the monitoring of patients with seminoma. Br J Urol. 1996. 77:138–142.

18. Lau SK, Weiss LM, Chu PG. D2-40 immunohistochemistry in the differential diagnosis of seminoma and embryonal carcinoma: a comparative immunohistochemical study with KIT (CD117) and CD30. Mod Pathol. 2007. 20:320–325.

19. Leroy X, Augusto D, Leteurtre E, Gosselin B. CD30 and CD117 (c-kit) used in combination are useful for distinguishing embryonal carcinoma from seminoma. J Histochem Cytochem. 2002. 50:283–285.

20. McCluggage WG, Shanks JH, Whiteside C, Maxwell P, Banerjee SS, Biggart JD. Immunohistochemical study of testicular sex cord-stromal tumors, including staining with anti-inhibin antibody. Am J Surg Pathol. 1998. 22:615–619.

21. McEntee K. McEntee K, editor. Scrotum, Spermatic cord and testis proliferative lesions. Reproductive Pathology of Domestic Mammals. 1990. San Diego: Academic Press;279–300.

22. McLachlan NJ, Kennedy PC. Meuten DJ, editor. Tumors of the genital system. Tumors in Domestic Animals. 2002. 4th ed. Ames: Iowa State University Press;561–573.
23. Miller MA, Hartnett SE, Ramos-Vara JA. Interstitial cell tumor and Sertoli cell tumor in the testis of a cat. Vet Pathol. 2007. 44:394–397.

24. Mooney EE, Nogales FF, Bergeron C, Tavassoli FA. Retiform Sertoli-Leydig cell tumours: clinical, morphological and immunohistochemical findings. Histopathology. 2002. 41:110–117.

25. Mostofi FK, Sesterhenn IA. Pathology of germ cell tumors of testes. Prog Clin Biol Res. 1985. 203:1–34.
26. Nakai Y, Nonomura N, Oka D, Shiba M, Arai Y, Nakayama M, Inoue H, Nishimura K, Aozasa K, Mizutani Y, Miki T, Okuyama A. KIT (c-KIT oncogene product) pathway is constitutively activated in human testicular germ cell tumors. Biochem Biophys Res Commun. 2005. 337:289–296.

27. Nikolaou M, Valavanis C, Aravantinos G, Fountzilas G, Tamvakis N, Lekka I, Arapantoni-Dadioti P, Zizi A, Ghiconti I, Economopoulos T, Pectasides D. KIT expression in male germ cell tumors. Anticancer Res. 2007. 27:1685–1688.
28. Owston MA, Ramos-Vara JA. Histologic and immunohistochemical characterization of a testicular mixed germ cell sex cord-stromal tumor and a leydig cell tumor in a dog. Vet Pathol. 2007. 44:936–943.

29. Pera MF, Herszfeld D. Differentiation of human pluripotent teratocarcinoma stem cells induced by bone morphogenetic protein-2. Reprod Fertil Dev. 1998. 10:551–555.

30. Peters MA, Teerds KJ, van der Gaag I, de Rooij DG, van Sluijs FJ. Use of antibodies against LH receptor, 3beta-h droxysteroid dehydrogenase and vimentin to characterize different types of testicular tumour in dogs. Reproduction. 2001. 121:287–296.
31. Rajpert-De Meyts E. Recent advances and future directions in research on testicular germ cell cancer. Int J Androl. 2007. 30:192–197.

32. Rosai J. Rosai and Ackerman's Surgical Pathology. 2004. 4th ed. Edinburgh: Mosby;1412–1456.
33. Sung MT, Jones TD, Beck SD, Foster RS, Cheng L. OCT4 is superior to CD30 in the diagnosis of metastatic embryonal carcinomas after chemotherapy. Hum Pathol. 2006. 37:662–667.

34. Taniyama H, Hirayama K, Nakada K, Numagami K, Yaosaka N, Kagawa Y, Izumisawa Y, Nakade T, Tanaka Y, Watanabe G, Taya K. Immunohistochemical detection of inhibin-alpha, -betaB, and -betaA chains and 3beta-ydroxysteroid dehydrogenase in canine testicular tumors and normal testes. Vet Pathol. 2001. 38:661–666.

35. Teng LH, Lu DH, Xu QZ, Fu YJ, Yang H, He ZL. Expression and diagnostic significance of OCT4, CD117 and CD30 in germ cell tumors. Zhonghua Bing Li Xue Za Zhi. 2005. 34:711–715.
36. Ulbright TM, Amin MB, Young RH. Tumors of the Testis, Adnexa, Spermatic Cord, and Scrotum. 1999. 3rd ed. Washington DC: Armed Forces Institute of Pathology;59–85.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download