Abstract
Objective
To determine which computed tomography (CT) imaging features predict pleural malignancy in patients with advanced epithelial ovarian carcinoma (EOC) using video-assisted thoracic surgery (VATS), pathology, and cytology findings as the reference standard.
Methods
This retrospective study included 44 patients with International Federation of Obstetrics and Gynecology (FIGO) stage III or IV primary or recurrent EOC who had chest CT ≤30 days before VATS. Two radiologists independently reviewed the CT studies and recorded the presence and size of pleural effusions and of ascites; pleural nodules, thickening, enhancement, subdiaphragmatic tumour deposits and supradiaphragmatic, mediastinal, hilar, and retroperitoneal adenopathy; and peritoneal seeding. VATS, pathology, and cytology findings constituted the reference standard.
Results
In 26/44 (59%) patients, pleural biopsies were malignant. Only the size of left-sided pleural effusion (reader 1: rho=-0.39, p=0.01; reader 2: rho=-0.37, p=0.01) and presence of ascites (reader 1: rho=-0.33, p=0.03; reader 2: rho=-0.35, p=0.03) were significantly associated with solid pleural metastasis. Pleural fluid cytology was malignant in 26/35 (74%) patients. Only the presence (p=0.03 for both readers) and size (reader 1: rho=0.34, p=0.04; reader 2: rho=0.33, p=0.06) of right-sided pleural effusion were associated with malignant pleural effusion. Interobserver agreement was substantial (kappa=0.78) for effusion size and moderate (kappa=0.46) for presence of solid pleural disease. No other CT features were associated with malignancy at biopsy or cytology.
Conclusion
In patients with advanced EOC, ascites and left-sided pleural effusion size were associated with solid pleural metastasis, while the presence and size of right-sided effusion were associated with malignant pleural effusion. No other CT features evaluated were associated with pleural malignancy.
Among gynecologic cancers, epithelial ovarian cancer (EOC) is second only to cervical cancer in incidence and number of deaths caused [1]. Patients with ovarian cancer are staged according to the International Federation of Obstetrics and Gynecology (FIGO) system [2], which determines patient management and provides prognostic information (five-year survival rates are 32.5% and 18.6% for stages IIIC and IV, respectively) [3]. Cytoreductive surgery is the treatment of choice for patients with ovarian cancer. "Optimal" cytoreductive surgery (residual disease <1 cm) is a very strong predictor of survival [4], and even after the threshold for optimal cytoreduction has been reached, it is important to remove as much of the residual tumor as possible [5].
Circulation of peritoneal fluid throughout the abdomen and pelvis commonly results in diaphragmatic tumor implants, and in turn, peritoneal-pleural communication through the diaphragm may allow trans-diaphragmatic spread of tumor into the thorax [6]. Malignant thoracic involvement indicates FIGO stage IV disease, for which treatment options include neoadjuvant chemotherapy and, in patients who are candidates for optimal abdominal cytoreduction, thoracic debulking [7,8]. Neoadjuvant chemotherapy followed by interval debulking surgery is a suitable alternative [9,10] that is supported by the outcome of a recent multicentre randomised controlled trial [11].
Several studies have demonstrated that the thorax frequently harbors undiagnosed pleural disease at the time of the initial diagnosis, and that this is likely to affect survival even in cases of optimal debulking [12-14]. In addition, a recent study found that moderate-to-large pleural effusion on preoperative computed tomography (CT) was associated with a decrease in overall survival in patients with stage III or IV EOC after controlling for age, preoperative CA-125, surgical stage, ascites, and cytoreductive status [15]. Therefore, accurate identification of the presence and extent of thoracic disease, including both solid metastasis and malignant pleural effusion, is important for determining prognosis and selecting appropriate treatment in patients with advanced EOC [15] (Figs. 1 and 2).
Pleural effusions in ovarian cancer have a higher likelihood of malignancy when they are moderate to large in size and associated with enlarged superior diaphragmatic lymph nodes [6]. However, studies on CT indicators of malignant pleural effusion have used cytology rather than surgical findings as the reference standard [6,16,17]. The accuracy of pleural fluid cytologic examination ranges between 40% and 87% [18-20]. Video-assisted thoracic surgery (VATS) has been shown to enable accurate pathological diagnosis and intrathoracic resection of pleural metastasis in patients with ovarian cancer [21]. In a small retrospective study from our institution, 4/10 patients (40%) with negative cytological findings had macroscopic pleural lesions on VATS [13], and in another such study, preoperative CT identified solid pleural disease in only one third of patients (2/6) who had macroscopic disease on VATS [14]. Therefore, the aims of our study were to compare chest CT to VATS, pathology, and cytology findings and determine possible CT imaging features that may be predictive of pleural malignancy in patients with advanced EOC.
Our retrospective, cross-sectional imaging study was compliant with Health Insurance Portability and Accountability Act. The institutional review board approved the study and issued a waiver of informed consent. Patients were selected by means of a computerized review of institutional gynecologic surgery and radiology databases. Consecutive patients with primary or recurrent FIGO stage III and IV ovarian cancer who underwent CT of the chest up to 30 days before VATS from January 1, 1997 through December 31, 2009 were included in the study. If a patient had more than one CT study within the 30 days before VATS, the most recent one was used. A total of 44 patients (3 with FIGO stage III and 41 with FIGO stage IV) were included in the analysis, of whom 41 had primary and 3 had recurrent disease. Indications for VATS were malignant pleural effusion on cytology (12/44), suspected malignant nodules or lymph nodes on CT (16/44) or moderate to large (defined as occupying 1/3 or more of the lung field on chest X-ray) and/or recurrent pleural effusions (16/44).
Most of the CT examinations (32/44) were performed with intravenous contrast medium, and the slice thickness ranged from 5 to 8 mm. For CT examinations performed outside our institution, the type of CT scanner and the amount of contrast material used were unknown. At our institution, CT was performed with various scanners (GE Medical Systems, Milwaukee, WI, USA). Our standard CT protocols were tailored to the individual scanners. Before October 1, 2000, studies were obtained with a conventional non-helical scanner; afterwards they were obtained with a helical scanner with one to 64 detector rows.
For all CT examinations performed at our institution, a dynamic power injection of 150 mL of nonionic intravenous contrast material was administered at a rate of 2.5 mL/second. Time delay to scanning varied with the type of scanner used but was determined on the basis of the typical time to portal venous phase imaging. All CT studies that were done outside our institution were digitized and sent to our enterprise-wide picture archiving and communication system.
Images were retrospectively and independently analyzed by two radiologists (SM and ES) who were aware that the patients had ovarian cancer but were blinded to the patients' clinical data, prospective CT reports, and pathology findings. Both readers were fellowship-trained in body imaging with 8 years of experience in oncologic imaging.
For each patient, the radiologists recorded the presence and size of pleural effusion. Pleural effusion size was categorized based on visual estimation as small (occupying less than 1/3 of the visualized hemithorax), moderate (1/3 to 2/3 of the hemithorax), or large (more than 2/3 of the hemithorax). Previous work has suggested visual approximation to be about 85% accurate compared to 3D CT reconstruction [22]. The radiologists recorded the presence of pleural nodules, pleural thickening (greater than 3 mm), pleural enhancement and subdiaphragmatic deposits. They also noted the presence of supradiaphragmatic (greater than 5 mm), mediastinal, hilar, and retroperitoneal lymphadenopathy. In addition, they estimated the size of ascites and recorded the presence of peritoneal seeding if present.
Results from VATS served as the reference standard. All procedures were performed at our institution by gynecologic oncology and thoracic surgical teams whose members' experience ranged from 5 to 25 years. VATS was performed based on CT findings. Right-sided VATS was performed in 27 of the 44 patients, left-sided VATS in 8 and bilateral in 9. The procedure included supradiaphragmatic lymph node dissection if indicated (in 12 patients), drainage of effusion (in 35 patients), and pleural biopsy of any suspicious areas or of random areas if no suspicious areas were identified. A research study assistant (OM) reviewed the intraoperative notes and pathology reports retrospectively to determine whether any effusion was drained preoperatively or intraoperatively and to record cytopathological and histopathological results (for 9 patients, cytology results were not available either because there was no effusion or because macroscopic pleural disease was present and thus cytologic evaluation was not completed, as it would not have been clinically relevant).
Differences in age were compared using the Wilcoxon ranksum test. Differences in imaging features between benign and malignant findings at cytopathology or histopathology were evaluated using Fisher's exact test and Spearman's rank correlation. Kappa statistics along with 95% confidence intervals were calculated to assess inter-reader agreement and were interpreted as follows: κ<0.00, no agreement; 0.00≤κ≤0.20, slight agreement; 0.21≤κ≤0.40, fair agreement; 0.41≤κ≤0.60, moderate agreement; 0.61≤κ≤0.80, substantial agreement; and 0.81≤κ≤1.0, almost perfect agreement. All p-values less than 0.05 were considered statistically significant. All statistical analyses were performed by using commercially available software (SPSS ver. 16.0, SPSS Inc, Chicago, IL, USA; SAS ver. 9.0, SAS Institute, Cary, NC, USA).
The patients' median age was 56 years (range, 40 to 81 years). The median time from CT to surgery was 7 days (range, 2 to 30 days). Age did not differ significantly between patients with and without solid pleural metastasis (p=0.66) or between patients with and without malignant pleural effusions (p=0.16).
In 26/44 patients (59%), pleural biopsies were positive for malignancy. For both readers, the size of left-sided pleural effusion (rho=-0.39, p=0.01 and rho=-0.37, p=0.01 for readers 1 and 2, respectively) and the presence of ascites (rho=-0.33, p=0.03 and rho=-0.35, p=0.03 for readers 1 and 2, respectively) were the only imaging features associated with malignant pleural involvement on CT, there was no difference in the presence of pleural nodules, pleural enhancement, pleural thickening, subdiaphragmatic deposits, supradiaphragmatic lymphadenopathy, mediastinal lymphadenopathy, retroperitoneal lymphadenopathy, hilar lymphadenopathy, supraclavicular lymphadenopathy or the presence of peritoneal seeding between patients with and without pleural malignancy at biopsy (Tables 1 and 2).
In 26 (74%) of the 35 patients who had pleural fluid cytology available, the results indicated malignant pleural effusion. The only features associated with malignant pleural effusion were the presence (p=0.03 for both readers) and size (rho=0.34, p=0.04 and rho=0.33, p=0.06 for readers 1 and 2, respectively) of any right-sided pleural effusion (Tables 1 and 2).
Interobserver agreement was substantial (κ=0.78) for pleural effusion size and moderate for presence of solid pleural disease (κ=0.46). Agreement on individual pleural findings varied from fair to substantial, with κ values of 0.21 for agreement on pleural nodules, 0.48 for agreement on pleural thickening, and 0.61 for agreement on pleural enhancement. Agreement on the amount of ascites was fair (κ=0.37).
The management of patients with malignant pleural effusions and/or intrathoracic metastasis from primary or recurrent advanced EOC is not standardized. The Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference statement, 2004 [23] and the evidence from randomized controlled trials [11,24] indicate that disease outside the peritoneum precludes optimal debulking and therefore these patients should undergo neoadjuvant chemotherapy followed by cytoreductive surgery. Still other studies have reported that patients with malignant pleural effusions as the only extraperitoneal manifestation of the disease have a more favorable prognosis than patients with other sites of stage IV disease such as liver or distant lymph nodes [25-27]. A study from our institution in patients who had optimal cytoreduction showed a worse prognosis for those with than for those without malignant pleural effusions [12]. Therefore, it is important for patients with pleural effusions to be appropriately triaged for intrathoracic cytoreduction or neoadjuvant chemotherapy depending on the feasibility of optimal cytoreduction [13,14,28-30].
In a very recent study of 203 patients with FIGO stage III and IV EOC who underwent CT before primary cytoreductive surgery at our institution, the presence of moderate-to-large pleural effusion on preoperative CT was independently associated with poorer overall survival (reader 1: hazard ratio [HR], 2.26, 95% confidence interval [CI], 1.31 to 3.91, p<0.01; reader 2: HR, 2.25, 95% CI, 1.26 to 4.01, p=0.02) after controlling for age, preoperative CA-125, surgical stage, ascites and cytoreductive status. Since many patients who had pleural effusions did not have the pleural fluid removed and evaluated for malignant cells, it was unclear if the poor prognosis associated with moderate-to-large pleural effusions was due to the size of the effusions, the likelihood that they were malignant, the possibility that they were associated with bulky intrathoracic disease, or a combination of the above. We have previously reported that as many as two-thirds of patients with pleural effusions have gross intrathoracic disease on VATS [14]. Therefore, at our institution VATS is now routinely performed in patients with moderate-to-large pleural effusions.
In our present study the size of pleural effusion and the presence of ascites on CT were associated with malignant pleural effusion. However, CT features generally considered suggestive of solid pleural malignancy such as solid pleural nodules, pleural thickening and enhancement were not associated with pleural malignancy at biopsy or cytology obtained during VATS. Our results agree with those of a prior study of 15 patients with advanced ovarian cancer, in which videoassisted thoracoscopy was done to evaluate unilateral or bilateral pleural effusions or (in one patient) to assess the effects of neoadjuvant chemotherapy on pleural metastases [31]. The study found that preoperative CT had sensitivity of just 14% and specificity of only 25% for determining pleural status when video-assisted thoracoscopy was used as the reference standard [31].
Several studies have investigated the use of positron emission tomography (PET) or PET/CT as an alternative imaging test for diagnosing pleural metastases in patients with mesothelioma and non-small-cell lung cancer; using pathology from VATS or cytology obtained from thoracocentesis as the reference standard. They found very high accuracy ranging from 92% to 97.5% [32-24]. In the primary staging of ovarian cancer, Kitajima et al. [35] found that integrated FDG-PET/contrast-enhanced CT was more accurate than CT alone. The incremental value of PET/CT in per-lesion accuracy was greater in extrapelvic sites, particularly metastatic lymph nodes in the abdomen; however, almost all patients (39/40) in the study had stage III disease [35]. Further studies are needed to assess the potential of PET/CT for evaluating thoracic metastases from ovarian cancer.
Our study had a number of limitations. First, selection bias may have affected our results, since patients had VATS because of suspicion of pleural malignancy based either on CT findings or pleural cytology. Optimally, the same study could be repeated with patients who routinely undergo VATS regardless of CT findings. Second, we chose an arbitrary period of ≤ 30 days between imaging and surgery. However, we reviewed only the most recent imaging study for each patient so the interval between CT examination and surgery would be as short as possible and the analysis of the relationship between CT and surgical findings would be valid. Third, we combined patients with stage III, stage IV, recurrent disease and different histological subtypes. Subgroup analysis was not feasible given the limited number of patients who underwent VATS. Finally, although the size and laterality of pleural effusion are unlikely to be affected by scanning technique or equipment, we cannot account for the variations in CT technique and equipment that occurred during the large timespan of this study. Detection of pleural metastasis could have been difficult on digitized images from other institutions, and this could account for the fact that inter-reader agreement was lower for the presence of solid pleural disease (κ=0.46) than for the size of pleural effusion (κ=0.78). Since a number of the CT examinations were performed at outside institutions it was not possible to apply a computer-assisted method of effusion volume estimation, which may be more accurate than the visual method that was used in our study.
Our study also had some important advantages compared to other published studies. First, to our knowledge, our sample size was larger than that of any other published study comparing VATS and CT findings of pleural disease in patients with advanced primary or recurrent EOC. Second, we relied on independent CT readings by two radiologists with substantial experience in oncologic imaging rather than consensus evaluation of CT images.
In conclusion, we found that in patients with advanced or recurrent epithelial ovarian cancer, CT imaging findings generally considered suggestive of malignant pleural involvement such as nodularity, thickening and enhancement of the pleura do not predict the presence of pleural malignancy on VATS. Therefore, VATS should be considered to evaluate thoracic involvement in ovarian cancer and facilitate appropriate management.
Figures and Tables
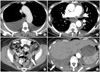 | Fig. 1Postmenopausal female with stage IV high-grade papillary serous carcinoma. (A) Contrast-enhanced computed tomography (CT) scan demonstrates bilateral pleural effusions (white arrows), larger on the right side, at the time of initial diagnosis. Right-sided pleural tap revealed positive cytology. Subsequently, bilateral video-assisted thoracic surgery was performed and revealed the presence of microscopic pleural metastasis bilaterally. Pleural effusions were treated with talc pleurodesis. (B) Post-treatment follow-up CT images demonstrate residual loculated small bilateral effusions (black arrow) and talc-related hyperdensity within the right pleural space (white arrow). (C) Contrast-enhanced CT scan 4 months after initial diagnosis and neoadjuvant chemotherapy showing residual disease in the abdomen and pelvis (white arrow) that was subsequently treated with optimal debulking. (D) Contrast-enhanced CT scan 6 months after optimal debulking showing gross pleural metastases (white arrows). |
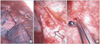 | Fig. 2Photographs obtained during video-assisted thoracic surgery show (A) white tumor plaques (white arrow) and collapsed lung (black arrow), (B) tumor plaques on the pleura (arrow), and (C) biopsy of pleural tumor. |
Table 1
Comparison of CT features in patients with and without pleural malignancy as assessed by reader 1

References
1. Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006. 20:207–225.
2. FIGO Committee on Gynecologic Oncology. Denny L, Hacker NF, Gori J, Johns HW III, Ngan HY, et al. Staging classifications and clinical practice guidelines for gynecologic cancers. 2000. Oxford: Elsevier.
3. Ozols RF. Update on Gynecologic Oncology Group (GOG) trials in ovarian cancer. Cancer Invest. 2004. 22:Suppl 2. 11–20.
4. Chi DS, McCaughty K, Diaz JP, Huh J, Schwabenbauer S, Hummer AJ, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006. 106:1933–1939.
5. Morgan RJ Jr, Alvarez RD, Armstrong DK, Boston B, Burger RA, Chen LM, et al. NCCN Clinical Practice Guidelines in Oncology: epithelial ovarian cancer. J Natl Compr Canc Netw. 2011. 9:82–113.
6. Kim KW, Choi HJ, Kang S, Park SY, Jung DC, Cho JY, et al. The utility of multi-detector computed tomography in the diagnosis of malignant pleural effusion in the patients with ovarian cancer. Eur J Radiol. 2010. 75:230–235.
7. Diaz JP, Abu-Rustum NR, Sonoda Y, Downey RJ, Park BJ, Flores RM, et al. Video-assisted thoracic surgery (VATS) evaluation of pleural effusions in patients with newly diagnosed advanced ovarian carcinoma can influence the primary management choice for these patients. Gynecol Oncol. 2010. 116:483–488.
8. Munstedt K, Franke FE. Role of primary surgery in advanced ovarian cancer. World J Surg Oncol. 2004. 2:32.
9. Huober J, Meyer A, Wagner U, Wallwiener D. The role of neoadjuvant chemotherapy and interval laparotomy in advanced ovarian cancer. J Cancer Res Clin Oncol. 2002. 128:153–160.
10. Schwartz PE. Cytoreductive surgery for the management of stage IV ovarian cancer. Gynecol Oncol. 1997. 64:1–3.
11. Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010. 363:943–953.
12. Eitan R, Levine DA, Abu-Rustum N, Sonoda Y, Huh JN, Franklin CC, et al. The clinical significance of malignant pleural effusions in patients with optimally debulked ovarian carcinoma. Cancer. 2005. 103:1397–1401.
13. Juretzka MM, Abu-Rustum NR, Sonoda Y, Downey RJ, Flores RM, Park BJ, et al. The impact of video-assisted thoracic surgery (VATS) in patients with suspected advanced ovarian malignancies and pleural effusions. Gynecol Oncol. 2007. 104:670–674.
14. Chi DS, Abu-Rustum NR, Sonoda Y, Chen SW, Flores RM, Downey R, et al. The benefit of video-assisted thoracoscopic surgery before planned abdominal exploration in patients with suspected advanced ovarian cancer and moderate to large pleural effusions. Gynecol Oncol. 2004. 94:307–311.
15. Mironov O, Ishill NM, Mironov S, Vargas HA, Zheng J, Moskowitz CS, et al. Pleural effusion detected at CT prior to primary cytoreduction for stage III or IV ovarian carcinoma: effect on survival. Radiology. 2011. 258:776–784.
16. Leung AN, Muller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol. 1990. 154:487–492.
17. Traill ZC, Davies RJ, Gleeson FV. Thoracic computed tomography in patients with suspected malignant pleural effusions. Clin Radiol. 2001. 56:193–196.
18. Grunze H. The comparative diagnostic accuracy, efficiency and specificity of cytologic technics used in the diagnosis of malignant neoplasm in serous effusions of the pleural and pericardial cavities. Acta Cytol. 1964. 8:150–163.
19. Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J. 1997. 10:1907–1913.
20. Sallach SM, Sallach JA, Vasquez E, Schultz L, Kvale P. Volume of pleural fluid required for diagnosis of pleural malignancy. Chest. 2002. 122:1913–1917.
21. Lim MC, Lee HS, Jung DC, Choi JY, Seo SS, Park SY. Pathological diagnosis and cytoreduction of cardiophrenic lymph node and pleural metastasis in ovarian cancer patients using video-assisted thoracic surgery. Ann Surg Oncol. 2009. 16:1990–1996.
22. Mergo PJ, Helmberger T, Didovic J, Cernigliaro J, Ros PR, Staab EV. New formula for quantification of pleural effusions from computed tomography. J Thorac Imaging. 1999. 14:122–125.
23. du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005. 16:Suppl 8. viii7–viii12.
24. van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer: Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1995. 332:629–634.
25. Bonnefoi H, A'Hern RP, Fisher C, Macfarlane V, Barton D, Blake P, et al. Natural history of stage IV epithelial ovarian cancer. J Clin Oncol. 1999. 17:767–775.
26. Penson RT, Skates SJ, Fuller AJ Jr, Seiden MV. Clinical course of stage IV epithelial ovarian cancer. J Clin Oncol. 1999. 17:3361–3362.
27. Aletti GD, Podratz KC, Cliby WA, Gostout BS. Stage IV ovarian cancer: disease site-specific rationale for postoperative treatment. Gynecol Oncol. 2009. 112:22–27.
28. Eisenkop SM. Thoracoscopy for the management of advanced epithelial ovarian cancer: a preliminary report. Gynecol Oncol. 2002. 84:315–320.
29. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002. 20:1248–1259.
30. Munkarah AR, Hallum AV 3rd, Morris M, Burke TW, Levenback C, Atkinson EN, et al. Prognostic significance of residual disease in patients with stage IV epithelial ovarian cancer. Gynecol Oncol. 1997. 64:13–17.
31. Cohen-Mouly S, Badia A, Bats AS, Barthes F, Bensaid C, Riquet M, et al. Role of video-assisted thoracoscopy in patients with ovarian cancer and pleural effusion. Int J Gynecol Cancer. 2009. 19:1662–1665.
32. Erasmus JJ, McAdams HP, Rossi SE, Goodman PC, Coleman RE, Patz EF. FDG PET of pleural effusions in patients with non-small cell lung cancer. AJR Am J Roentgenol. 2000. 175:245–249.
33. Orki A, Akin O, Tasci AE, Ciftci H, Urek S, Falay O, et al. The role of positron emission tomography/computed tomography in the diagnosis of pleural diseases. Thorac Cardiovasc Surg. 2009. 57:217–221.
34. Yildirim H, Metintas M, Entok E, Ak G, Ak I, Dundar E, et al. Clinical value of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiation of malignant mesothelioma from asbestos-related benign pleural disease: an observational pilot study. J Thorac Oncol. 2009. 4:1480–1484.
35. Kitajima K, Murakami K, Yamasaki E, Kaji Y, Fukasawa I, Inaba N, et al. Diagnostic accuracy of integrated FDG-PET/contrastenhanced CT in staging ovarian cancer: comparison with enhanced CT. Eur J Nucl Med Mol Imaging. 2008. 35:1912–1920.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download