This article has been
cited by other articles in ScienceCentral.
Abstract
Portal vein thrombosis (PVT) is a rare but serious postoperative complication of pancreaticoduodenectomy (PD). We reported a case of late-onset postoperative PVT with hemorrhage from the common hepatic artery (CHA) in a 73-year-old man who underwent pylorus-preserving pancreaticoduodenectomy (PPPD) for duodenum papilla cancer, followed by reconstruction using the modified Child's technique. The pancreaticojejunostomy was achieved by end-to-side, 2-layer invagination anastomosis without pancreatic duct stenting. Drain removal and hospital discharge were scheduled on postoperative day (POD) 18, but blood-stained fluid in the drain and sudden hematemesis were noted. Emergency surgery was performed because PVT and imaging findings were suggestive of necrosis of the lifted jejunum. Although no jejunal necrosis was identified during surgery, bleeding from the side of the CHA was detected and the bleeding point was suture-closed to achieve hemostasis. We suspected late-onset postoperative arterial hemorrhage and subsequent hematoma formation, which caused portal vein compression and PVT formation. We chose a conservative treatment strategy for PVT, taking into account the operation time, intraoperative vital signs and blood flow in the portal vein. Despite the complicated postoperative course, he was discharged home in a fully ambulatory state on POD 167.
Go to :

Keywords: Portal vein thrombosis, Pancreaticoduodenectomy, Late-onset hemorrhage, Postoperative hemorrhage, Postoperative pancreatic fistula
INTRODUCTION
Pancreaticoduodenectomy (PD) is a standard surgical technique used for the resection of a tumor in the head of the pancreas. Typical postoperative complications of PD include postoperative pancreatic fistula (POPF), delayed gastric emptying, intra-abdominal hemorrhage and intra-abdominal abscess. We experienced a rare and challenging case of postoperative delayed bleeding from the common hepatic artery (CHA), portal vein thrombosis (PVT) and gastric hemorrhage following pylorus-preserving pancreaticoduodenectomy (PPPD). To our knowledge, this was a rare case
123 of PVT as a postoperative complication of PD in the absence of predisposing factors such as portal reconstruction, portal hypertension and irradiation.
Go to :

CASE
A 73-year-old man underwent PPPD for duodenum papilla cancer that was discovered during screening for impaired liver function. Closed-circuit continuous low-pressure suction was placed at the pancreaticojejunostomy and the Winslow foramen. Amylase level of the drain from the pancreaticojejunostomy was 1783 IU/L on post-operative day (POD) 3, which was 3 times higher than the upper limit of normal of serum amylase level and thus indicated the presence of POPF according to the International Study Group on Pancreatic Fistula (ISGPF) classification.
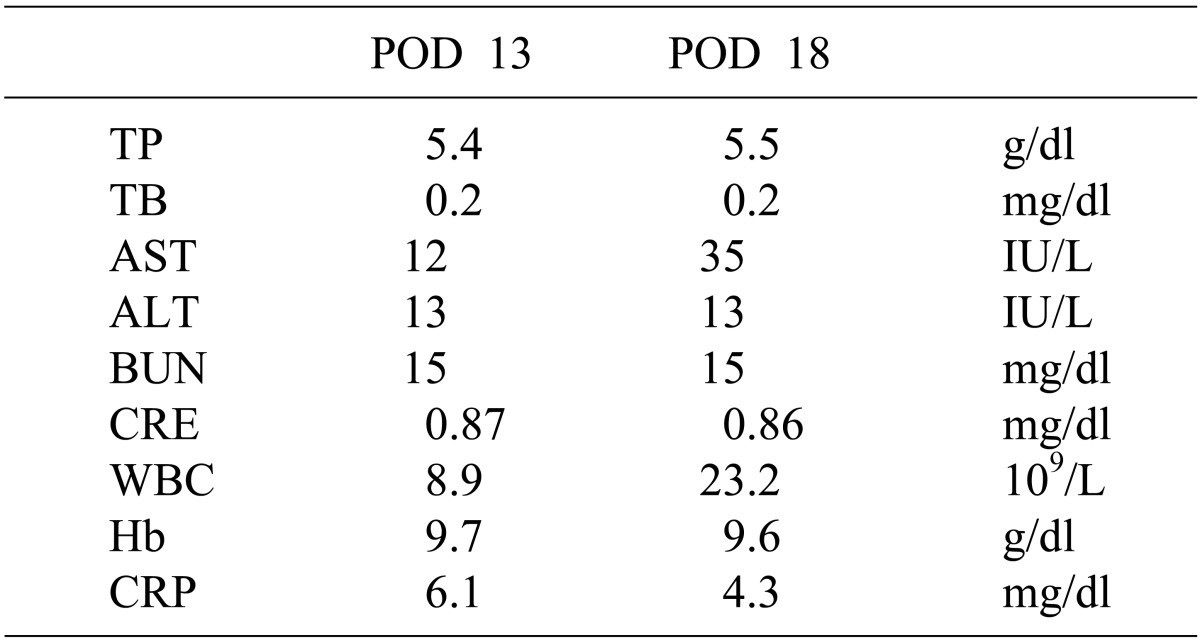
4 With effective drainage at the pancreaticojejunostomy and the Winslow foramen, POPF did not affect the patient's general condition. The drain amylase level reached the serum normal range on POD 10. Drain removal and discharge from hospital were considered on POD 18 due to no other complications; however, blood-stained fluid in the drain was noted and sudden hematemesis of 1500 ml blood occurred during plane computed tomography (CT) scanning (
Table 1). Upper gastro-intestinal endoscopy revealed erosion in the upper posterior wall of the gastric corpus and bleeding from the lesion. A soft coagulation device was used to achieve hemostasis. Plane CT showed gas in the superior mesenteric vein (SMV) and intramural emphysema of the lifted jejunum (
Fig. 1). Contrast-enhanced CT at POD 18 showed no evidence of leakage of contrast agent in the abdominal cavity but PVT was most prominent around the bifurcation of the SMV and splenic vein. Portal blood flow was preserved (
Fig. 2). However, emergency surgery was performed on the same day because necrosis of the lifted jejunum was suspected.
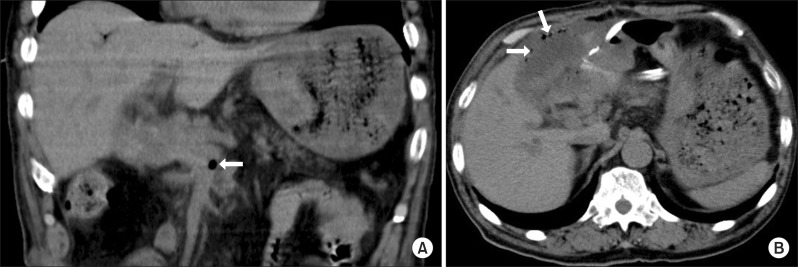 | Fig. 1Abdominal computed tomography (CT) scans at postoperative day 18 showed edematous wall thickening and portal vein gas associated with bowel ischemia and necrosis. (A) Gas in the superior mesenteric vein (arrows); (B) Gas in the area corresponding to the wall of the lifted jejunum, with a series of bubbles suggestive of intramural emphysema (arrows).
|
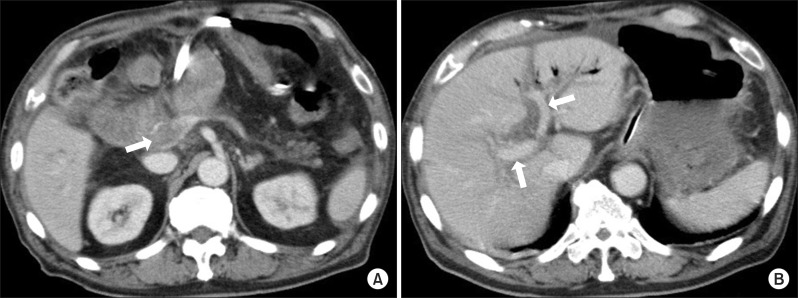 | Fig. 2Contrast-enhanced CT scans after hemostatic treatment at postoperative day 18. (A) Thrombi were found from the superior mesenteric vein to the portal vein (arrow); (B) The right and left intra-hepatic portal branches were contrast-enhanced, indicating preserved blood flow (arrows).
|
Table 1
Comparison of laboratory data

Intraoperatively, a moderate amount of bloody ascites and hematoma around the portal vein were noted but no intestinal discoloration suggestive of jejunal necrosis was observed. Triple-ligated, cut ends of the gastroduodenal artery and right gastric artery from the initial surgery were found on further exploration of the abdominal cavity. We further examined the abdominal cavity and identified the portion of the CHA just after its divergence from the celiac artery and where it is separated from the pancreas; the arterial wall was ruptured along about one-third of its circumference along the short axis, causing arterial bleeding. The CHA was clamped and suture-closed using 11 stitches with 5-0 vascular suture to achieve hemostasis. The portal vein thrombosis was managed conservatively.
After surgery, a tracheostomy was made for artificial respiration and anti-coagulation therapy was continued, with daily monitoring of blood flow in the hepatic artery and portal vein by ultrasonography (
Fig. 3). Ascites was managed with volumes up to 7000 ml per day; however, it decreased to about half the initial volume on POD 20, and was below 1000 ml/day 1 month after surgery. Hepatic functions were normalized after the second surgery. Contrast-enhanced CT scans taken immediately before hospital discharge revealed no remarkable change in residual PVT but the hepatic artery and intrahepatic portal branches could be clearly visualized. The patient recovered and was subsequently discharged in a fully ambulatory state on POD 167.
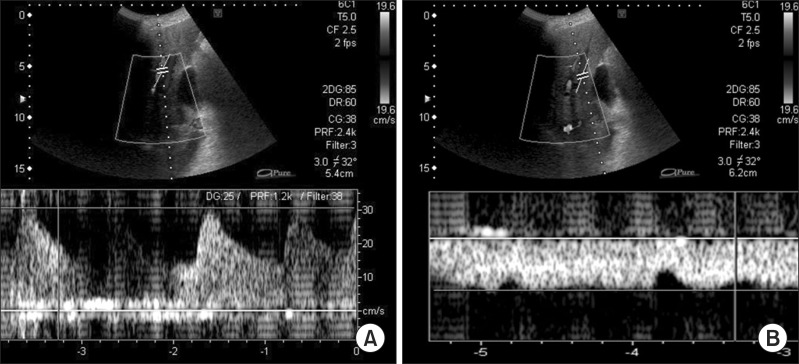 | Fig. 3Ultrasonographic examination of blood flow at 149 days after the second surgery. (A) Examination of the left hepatic artery was performed, as it was easily identified. The results confirm preserved blood flow; (B) Portal blood flow was easily measured at the umbilical point. The results confirm preserved blood flow.
|
Go to :

DISCUSSION
PD was first reported by Whipple et al.
5 in 1935 and has since been widely used as a standard surgical procedure primarily for tumors in the head of the pancreas. Although it is generally recognized that PD, with an 80-year history, can now be performed safely,
6 the procedure is still associated with a high postoperative complication rate of 23-38%.
789101112 Typical complications include POPF, delayed gastric emptying, and hemorrhage in the early postoperative period, while late-onset complications are often associated with reduced residual pancreatic function. We experienced a case of grade-A POPF, according to the ISGPF classification, after PPPD for duodenum papilla cancer, followed by late-onset complications including bleeding from the CHA, PVT and gastric hemorrhage. PVT as a postoperative complication of PD is very unusual. Combined resection of the portal vein or SMV may be selected for curative resection of the tumor, depending on the location and degree of invasion. In such cases, portal vein reconstruction may cause postoperative PVT. Other conditions associated with postoperative PVT include portal hypertension due to underlying hepatic cirrhosis and intraoperative irradiation. Our patient had no hepatic cirrhosis and did not undergo portal vein reconstruction or intraoperative irradiation. In 1964, Monge et al.
1 reported 3 patients with PVT who had undergone PD at the Mayo Clinic from their 22 years of experience. More recently, Thomas and Ahmad
3 reviewed post-operative PVT in 2010. As with any venous thrombotic condition, the etiology of acute PVT could be categorized based on Virchow's triad
13 of venous stasis, hypercoagulable state and endothelial injury.
Our patient had clinical symptoms of intra-abdominal hemorrhage and hematemesis, CT findings suggestive of necrosis of the lifted jejunum, gas in the portal vein and PVT, and upper gastro-intestinal endoscopic finding of hemorrhagic gastric erosion. We speculated that these conditions developed in the following order: arterial hemorrhage from the ruptured CHA; hematoma formation and subsequent compression of the portal vein; PVT formation due to compromised portal vein blood flow; and gastro-intestinal mucosal damage caused by disturbed venous perfusion to the portal vein. Because POPF, which was rated as grade A, improved along with inflammatory response before POD 10, it is unlikely that the formation of PVT was the direct effect of POPF. Venous stasis was considered a cause of PVT. There were no arterial branches that were ligated at the site during the initial surgery. A possible cause of the initial arterial hemorrhage was damage to the arterial adventitia caused by the dissection procedure, complicated by POPF. Because of the late-onset arterial bleeding, hematoma was localized by surrounding adhesion and strongly compressed the portal vein.
Other possible causes of PVT included thrombotic diathesis, but no abnormalities suggestive of antiphospholipid syndrome were detected, with an anti-CL/beta2-GPI antibody level of 1.2 U/ml (normal range: ≤3.499), anti-cardiolipin antibody level of 8 U/ml (≤9.9) and lupus anticoagulant level of 1.02 (<1.299). Although infusion therapy was completed on POD 7, it is unlikely that this led to dehydration, as the patient consumption was >80% of the served amount of rice gruel, drain discharge was <100 ml/day, and there was absence of hematologic findings suggestive of intravascular dehydration. PPPD was performed according to the standard procedure without portal vein reconstruction and was not accompanied by any special events associated with peri-portal dissection.
The optimal strategy for treating PVT is controversial. In the present case, as much as 7830 ml of intraoperative blood loss from arterial hemorrhage occurred during the second surgery, leading to significant hemodynamic instability. We did not perform portal thrombectomy and instead chose a conservative treatment strategy, given the excellent blood flow in the proper hepatic artery after repair of the CHA, preserved portal blood flow confirmed by ultrasonography and contrast-enhanced CT. However, it resulted in massive drain discharge after surgery and significant disturbances in electrolytes/protein levels and fluid balance.
Despite these considerations, postoperative hemorrhage and subsequent hematoma formation around the portal vein could lead to secondary PVT formation despite lack of disturbances in portal blood flow before or during surgery. Conservation for PVT resulted in massive discharge of up to 7000 ml/day after surgery and significant disturbances in electrolytes/protein levels and fluid balance. Concurrent and rapid development of fatal complications such as late-onset intra-abdominal hemorrhage and PVT formation within a short period of time are very serious consequences.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download