Abstract
Undifferentiated Embryonal Sarcoma of the Liver (UESL) is a tumor highly malignant, of mesenchymal origin. It is a rare finding in adults, though less rare in children. The strategy to be followed and the therapeutic targets to be reached for this tumor, in adult cases, remain ambiguous and controversial. Herein we report the case of a 29 year old female patient with a massive UESL and we describe our therapeutic approach. A 29 year-old female patient was referred to our center with severe intermittent epigastric pain and fever due to a voluminous liver tumor: Needle biopsy was of no specific findings and surgical excision was decided. Right portal vein embolization and selective embolization of the segment's IV branch was performed in order to achieve adequate future liver remnant (FLR). Right trisectonectomy was then performed, with uneventful post operative period and the patient was discharged at the 11th post operative day. UESL is a rare tumor that needs aggressive surgical approach and multidisciplinary team management is of paramount importance.
Go to : 
Sarcomas of the liver are tumors of low prevalence among population. Their classification is distinguished in angiosarcomas, leiomyosarcomas, fibrosarcomas, epitheloid hemangioendotheliomas, malignant fibrous histiocytomas and undifferentiated embryonal sarcomas of the liver (UESL). Undifferentiated Embryonal Sarcoma of the Liver (UESL) is of mesenchymal origin, with increased degree of malignancy. A thorough search through Medline (MeSH: Undifferentiated Embryonal Sarcomas Liver [All Fields] AND ("adult" [MeSH Terms] OR "adult" [All Fields]) brings out a limited number of papers that account for 68 cases, dating from 1955 to 2007. UESL as a clinical entity was first reported by Stocker and Ishak,1 in 1978 as a report of 31 cases.
Today it is considered mostly a childhood finding, ranging to ages from 6 to 10 years. When it comes to adults, its prevalence is lower, with approximately ten cases of primary UESL reported so far. The clinical treatment and strategy, for this tumor to be followed remains ambiguous, for adult patients. Therefore, we present an adult case of UESL that has been referred to our center.
Go to : 
A 29-year old female patient presented with severe intermittent epigastric pain that was irradiating to the right hypochondrium. Fever was also reported, of nonspecific character. The patient stated a loss of 5 kg during the last 15 days. She presented with no signs of liver deficiency, and in addition no jaundice, spider naevi or palmar erythema were present. In the past, she had been subjected to surgery for the removal of ovarian cysts in 2002 and 2003. No allergies, medications or other health issues were being raised. Laboratory findings were unremarkable and alpha-fetoprotein (AFP) values were normal. Chest X-ray failed to detect anything abnormal. Computed tomography (CT) scan revealed the presence of a voluminous space-occupying lesion, located to the right lobe of the liver. The mass was well circumscribed, with thickened periphery and some diaphragmatic separations inside. It was of low density and after the intravenous infusion of contrast, late enhancement was being presented. Additional magnetic resonance imaging showed the lesion mainly hypointense on T1 with hyperintensed foci that were ascribed to points of hemorrhage. On T2, the tumor was hyperintense. No lymph nodes were being detected. An ultrasonography-guided biopsy with fine needle was decided. Tumor biopsy revealed the presence of multiple inflammatory cells, and necrotic elements. Moreover, it was positive for neoplastic necrosis.
After a multi-disciplinary team (MDT) meeting, it was decided to proceed with a right trisectonectomy. Volumetry of the liver revealed a future liver remnant (FLR) of 32%. Since the FLR was considered insufficient for direct operation it was decided to perform embolization of the right portal vein, along with selective embolization of the portal branch to segment IV. Four weeks later, a new CT volumetry of the liver revealed hyperplastic growth in size of the FLR from 32% to 45%, and it was decided to proceed with a right trisectionectomy (Fig. 1).
The operation was a straightforward right trisectionectomy, a bilateral subcostal incision was performed and skeletonization resection of the hepatoduodenal ligament, including dissection of the regional lymph nodes, was performed from the duodenum to the liver. The right hepatic artery was divided. Then, at a more superior level, the right portal vein was divided, and was closed with a continuous suture. The right liver was mobilized and the right and middle hepatic veins were dissected and ligated with an EndoGIA, and right trisectionectomy was performed. Operation time was about 4 hours and total blood lose was 450 ml. No red blood cells were transfused, either during the operation or postoperative. The post-operative period was uneventful and the patient was discharged at the 11th postoperative day.
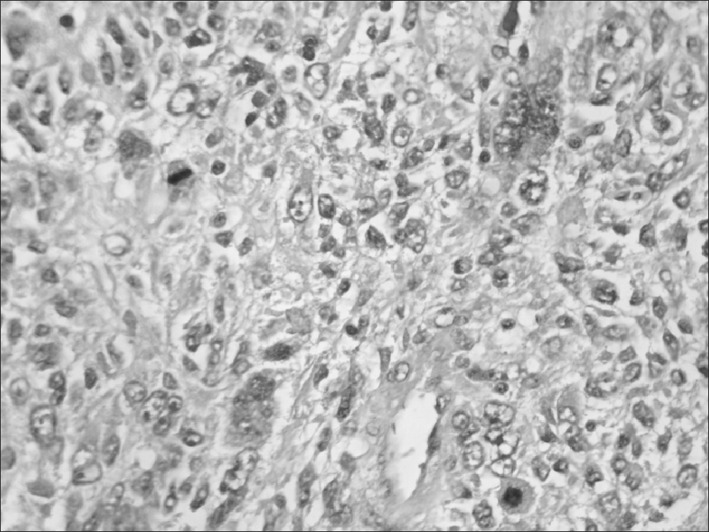
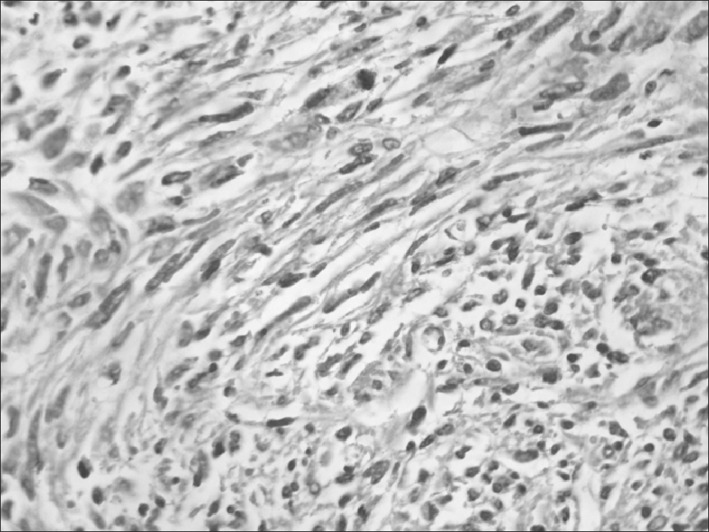
From the pathology report the tumor was positive for malignancy of mesenchymal character possibly vascular derived. The tumor was subcapsular, sharply demarcated from the surrounding liver parenchyma, solid, measured 18 cm in diameter, with grey-white glistening cut surface altering with cystic gelatinous areas and areas of hemorrhage and necrosis. Microscopically there were large areas of necrosis. In the viable areas the tumor cells were ovoid or spindle-shaped, with ill-defined outlines, often pleiomorphic, bizarre, with irregular, hyperchromatic nuclei, numerous mitoses and sometimes giant cells (Figs. 2 and 3). Multiple eosinophilic globules, Periodic acid–Schiff (PAS)-positive and diastase resistant were seen. The tumor cells arranged loosely in a myxoid matrix or more compactly in a fibrous stroma. At the periphery of the tumor trapped bile ducts and hepatocytes were detected. By immunohistochemistry the tumor cells were positive in vimentin, (mesenchymal marker) and weakly positive in desmin, a specific marker for myogenic differentiation. All of the following immunohistochemical markers were negative: S-100 protein and CD 57 (markers for schwannian differentiation), sarcomeric actin, myogenin, laminin, collagen IV (peripheral nerve sheeth tumor markers), c-kit (marker for GIST), Melan A and HMB 45 (melanocytic markers), CD 34 (endothelial marker), EMA and AE1/AE3 (epithelial markers), AFP and HepPar1 (hepatocytic markers). The diagnosis of an undifferentiated embryonal sarcoma of the liver (UESL) was made.
The patient was referred to the oncologists for adjuvant chemotherapy, and thirty cycles of combination of cyclophosphamide, cisplatin and doxorubicin were administered.
Follow up imaging six months after the operation revealed compensating left lobe hyperplasia and caudate lobe, homogenous liver parenchyma and absence of lymph nodes of pathological size. Twelve months after the operation a new CT scan revealed recurrence of the tumor to the liver. After a new MDT meeting, it was decided to continue the chemotherapy. A new CT scan 6 months after revealed total regression of the recurrence. The patient remained disease free for a few months when a new recurrence to the liver and metastasis to spine was presented. The patient died 28 months after the operation.
A written consent on the use of the material needed for this case report was being obtained by the family of the patient, following the directives of the University Ethics Committee, according to the 1975 declaration of Helsinki.
Go to : 
By now, UESL is considered a rare entity for adults. In child population, medical literature so far reports no more than 150 cases.2 UESL is listed fourth in the prevalence of primary hepatic tumors. Under the age of 18 years, the most common primary hepatic neoplasm is hepatoblastoma, and then comes hemangiendothelioma and hepatocellular carcinoma.3 It has to be added that there is no gender predominance, and it is evident a preference for the right hepatic lobe location.4
The histogenesis of UESL remains unknown.5 Despite its name there is not any resemblance with embyonal liver. The diffuse pattern of infiltration that tends to spare bile ducts provides the resemblance to mesenchymal hamartoma, which is now established as a precursor to the malignancy.
As a distinguished clinical pathology it was first described by Stocker and Ishak.1 in 1978. 31 cases were being presented. The prognosis was poor and the average survival was no more than 12 months. To the same report, only 6 out of 31 patients remained disease free after the surgical resection. Bisogno et al.,6 The Soft Tissue Sarcoma Italian and German Cooperative Groups presented a group of 4 patients who underwent combined treatment of surgical resection and then adjuvant therapy. Total remission was achieved for all of them, over a follow up from 5 to 10.9 years. The adjuvant treatment received by this group was a combination of vincristine, actinomycin, fosfamide and doxorubicine, as first line treatment. A disease-free follow up was also being described by Kim et al.7 In a group of three children that underwent the same treatment of surgical resection and adjuvant therapy mentioned above, they had no sign of tumor recurrence over a time of 40 to 60 months.7
Lenze et al.8 reviewed reports from 1995 to 2007, pointing out a significant predominance in survival if the treatment strategy included the combination of surgical resection and adjuvant chemotherapy. He also showed the absence of a study analyzing UESL treatment strategy and prognosis, not even in children. The report is a retrospective study comparing groups that have been treated with total surgical excision without adjuvant therapy, total surgical excision with adjuvant therapy and non-complete surgical excision with adjuvant chemotherapy. Their findings are clear; combining complete excision with adjuvant chemotherapy strongly dictates better prognosis and longer survival without complications.
Align with this result is the outcome of Almogy et al.9 In this report, with statistic findings from a single center, the superiority of adjuvant therapy versus no chemotherapy at all, is evident. To this conclusion has to be added the fact that these kinds of tumors augment their size without special signs or characteristic symptoms. When they are found, their volume is too large thus adjuvant therapy seems proper and rational. In a single report, García–Bonafé et al.10 highlight the value of the recognition of the cytologic features, through fine needle aspiration, of this type of tumor, in order to obtain a more targeted strategy on neoadjuvant therapy. It is also important to differentiate the tumor from a benign entity, when the imaging does not provide sufficient evidence.11
Taking this observation even further, Kim et al.7 along with the single center results reported by Ismail et al.,12 showed that neo-adjuvant therapy could lead to a diminished tumor size, making possible a complete surgical excision. Even if the tumor is excised in an oncological accepted surgical excision, without remnants (R0 resection), local recurrence along with metastases is not an uncommon finding.13 Repeated operations may be needed.14 Since there is no special serum marker that indicating recurrence, additional periodic imaging control is necessary post-operational.15
Our patient presented no augmented AFP, or any signs of cirrhosis, thus diminishing the possibility of a hepatocellular Carcinoma case. The absence though of specific findings from the needle biopsy did not permit the application of neo-adjuvant therapy to our strategy.
Combining all of the above, the recent literature and our center's experience, and since the diagnosis was obtained after operation, the patient was treated with 30 cycles of adjuvant therapy. Nevertheless, recurrence was aggressive, and the patient passed away 28 months after the operation.
Go to : 
References
1. Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978; 42:336–348. PMID: 208754.
2. Iqbal K, Xian ZM, Yuan C. Undifferentiated liver sarcoma--rare entity: a case report and review of the literature. J Med Case Rep. 2008; 2:20. PMID: 18218141.

3. Cao Q, Ye Z, Chen S, Liu N, Li S, Liu F. Undifferentiated embryonal sarcoma of liver: a multi-institutional experience with 9 cases. Int J Clin Exp Pathol. 2014; 7:8647–8656. PMID: 25674229.
4. Gao J, Fei L, Li S, Cui K, Zhang J, Yu F, et al. Undifferentiated embryonal sarcoma of the liver in a child: a case report and review of the literature. Oncol Lett. 2013; 5:739–742. PMID: 23426588.

5. Xu HF, Mao YL, Du SD, Chi TY, Lu X, Yang ZY, et al. Adult primary undifferentiated embryonal sarcoma of the liver: a case report. Chin Med J (Engl). 2010; 123:250–252. PMID: 20137381.
6. Bisogno G, Pilz T, Perilongo G, Ferrari A, Harms D, Ninfo V, et al. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer. 2002; 94:252–257. PMID: 11815984.
7. Kim DY, Kim KH, Jung SE, Lee SC, Park KW, Kim WK. Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg. 2002; 37:1419–1423. PMID: 12378446.

8. Lenze F, Birkfellner T, Lenz P, Hussein K, Länger F, Kreipe H, et al. Undifferentiated embryonal sarcoma of the liver in adults. Cancer. 2008; 112:2274–2282. PMID: 18361435.

9. Almogy G, Pappo O, Gips M, Lieberman S, Edden Y, Eid A. Improved survival with surgery and systemic chemotherapy for undifferentiated embryonal sarcoma of the liver. Isr Med Assoc J. 2005; 7:672–673. PMID: 16259353.
10. García-Bonafé M, Allende H, Fantova MJ, Tarragona J. Fine needle aspiration cytology of undifferentiated (embryonal) sarcoma of the liver. A case report. Acta Cytol. 1997; 41(4 Suppl):1273–1278. PMID: 9990257.
11. Xie ZY, Li LP, Wu WJ, Sun DY, Zhou MH, Zhao YG. Undifferentiated embryonal sarcoma of the liver mistaken for hepatic abscess in an adult. Oncol Lett. 2014; 8:1184–1186. PMID: 25120683.

12. Ismail H, Dembowska-Bagińska B, Broniszczak D, Kaliciński P, Maruszewski P, Kluge P, et al. Treatment of undifferentiated embryonal sarcoma of the liver in children--single center experience. J Pediatr Surg. 2013; 48:2202–2206. PMID: 24210186.

13. Dai CL, Xu F, Shu H, Xu YQ, Huang Y. Undifferentiated (embryonal) sarcoma of liver in adult: a case report. World J Gastroenterol. 2005; 11:926–929. PMID: 15682496.

14. Ma L, Liu YP, Geng CZ, Tian ZH, Wu GX, Wang XL. Undifferentiated embryonal sarcoma of liver in an old female: case report and review of the literature. World J Gastroenterol. 2008; 14:7267–7270. PMID: 19084947.

15. Li XW, Gong SJ, Song WH, Zhu JJ, Pan CH, Wu MC, et al. Undifferentiated liver embryonal sarcoma in adults: a report of four cases and literature review. World J Gastroenterol. 2010; 16:4725–4732. PMID: 20872975.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download