Abstract
Backgrounds/Aims
Although perioperative therapies have improved greatly, pancreatectomies still often need blood transfusions. However, the morbidity from blood transfusions, the poor prognosis of blood transfused patients, high cost, and decreasing supply of blood products is accelerating transfusion-free (TF) surgery in the patients who have pacreatectomies. The aim of this study was to assess the feasibility of TF pancreatectomies for patients who are Jehovah's Witness.
Methods
We investigated the possibility of TF pancreatectomies for the Jehovah's Witness patients undergoing pancreatectomies between January 2007 and Februay 2014. There were 4 cases of Whipple's operation, 4 of pylorus-preserving pancreaticoduodenectomy, 2 of radical antegrade modular pancreatosplenectomy and 1 of laparoscopic distal pancreatectomy. All were performed by one surgeon.
Results
Most of the TF pancreatecomies patients received perioperative blood augmentation and intraoperative acute normovolemic hemodilution (ANH). They received no blood transfusions at any time during their hospitalization, and pre- and intra-operative data and outcomes were acceptably favorable.
Conclusions
To the best of our knowledge, this report is the first successful consecutive pancreatectomy program for Jehovah's Witness not involving blood transfusion. TF pancreatectomy can be performed successfully in selected Jehovah's Witness. Postoperative prognosis and outcomes should be confirmed in follow up studies.
Go to : 
TF surgery has been gaining increased attention because of concern about viral infections.1234 There has been a persistent lack of blood products, and the lack of alternatives products has promoted TF surgeries even in hepatobiliary surgery including liver transplantation.56789
Pancreatic surgery, itself, has a relatively high chance of transfusion risk compared to other types of surgery mainly because most pancreas operations require challenging and complex operative techniques. Over the past several decades, there have been many advances in the operative techniques required in complex pancreatic surgery, yet despite technical improvements resulting in reduced need for blood product, allogenic blood transfusions are still required in many cases. According to a recent report based on the public use files of the American College of Surgeons National Surgical Quality Improvement Program, about 25.7% of hepatobiliary-pancreatic patients received more than one unit of red cells during the perioperative period. There were substantial differences in the incidence of transfusion between patients undergoing different types of pancreatectomy (distal pancreatectomy 19.8%, Whipple 28.7%, total pancreatectomy 43.6%).10 Recently, data for patients who underwent pancreatectomy and identified themselves as JW were published by the Memorial Sloan-Kettering Cancer Center. In that report, the median level of estimated blood loss (EBL) in the the pancreatectomies was 400 ml (range 250 to 1,800 ml). The findings showed that preoperative optimization of hemoglobin by blood augmentation achieved by intraoperative blood-conservation techniques, operative procedures minimizing blood loss and attentive postoperative care, permit the performance of major pancreatectomies in JW patients with good results.11 In recent reports published in Korea, intraoperative transfusion was required in 57% of pancreaticoduodenectomies,12 and 31% of laparoscopic pylorus-preserving pancreaticoduodenectomies.13 As mentioned above, although suitable non-TF techniques and materials have been developed, transfusion is still required in a substantial rumber of pancreatic operations. In this study, we report the clinical outcomes of the first successful program of pancreatectomies for JW patients suffering from pancreatic and peripancreatic lesions using a treatment algorithm for transfusion-refusing patients.
Go to : 
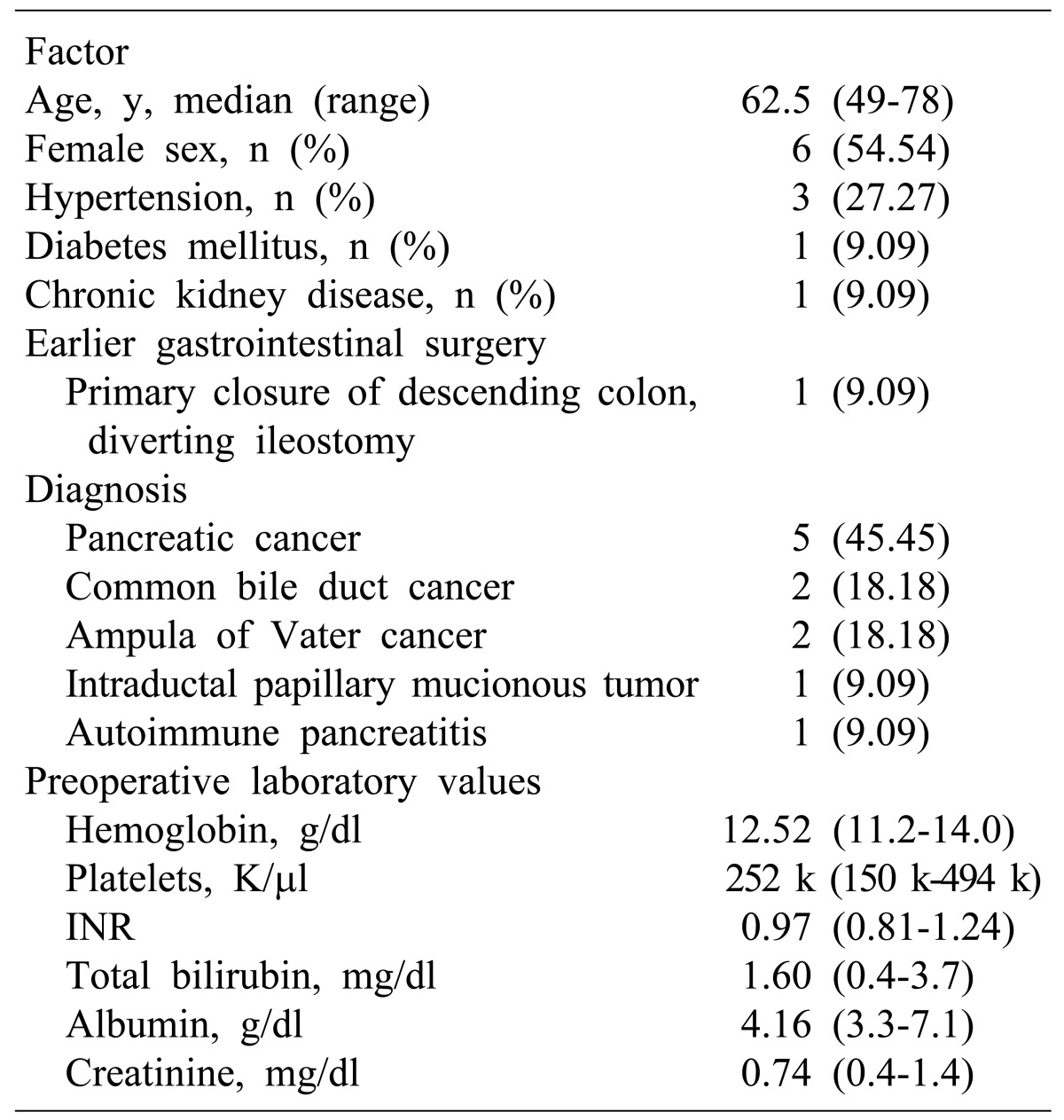
From July 2007 to Februrary 2014, 11 consecutive patients who underwent pancreatectomies were enrolled for this study from Soonchunhyang and Hanyang University Hospital. We have an active program of TF medicine and surgery. Every patient who underwent transfusion free pancreatectomy in this study period was informed about TF surgery. The pathological diagnosis of the individuals in this study were common pancreatic cancers (pancreatic ductal adenocarcinoma) (n=5, 45.45%), bile duct cancers (n=2, 18.18%), ampulla of Vater cancers (n=2, 18.18%), intraductalpapillary mucinous tumor (IPMT) (n=1, 9.09%), and 1 (9.09%) autoimmune pancreatitis. Of the total of 11 patinets, 5 were men and 6 women. Ages ranged 49 to 78 years (mean, 62.5 years). A varity of pancreatectomies were performed: pylorus-preserving pancreaticoduodenectomy (PPPD), standard pancreaticoduodenectomy, radical antegrade modular pancreaticosplenectomy (RAMPS), and laparoscopic distal pancreatectomy. From the beginning of the study, most of the relevant data on patients and operations were recorded in a computerized database, and additional data were obtained from the hospital charts. The hospital charts reviewed consisted of medical records, data on radiologic examinations, and histopathologic results. These records were analyzed restrospectively.
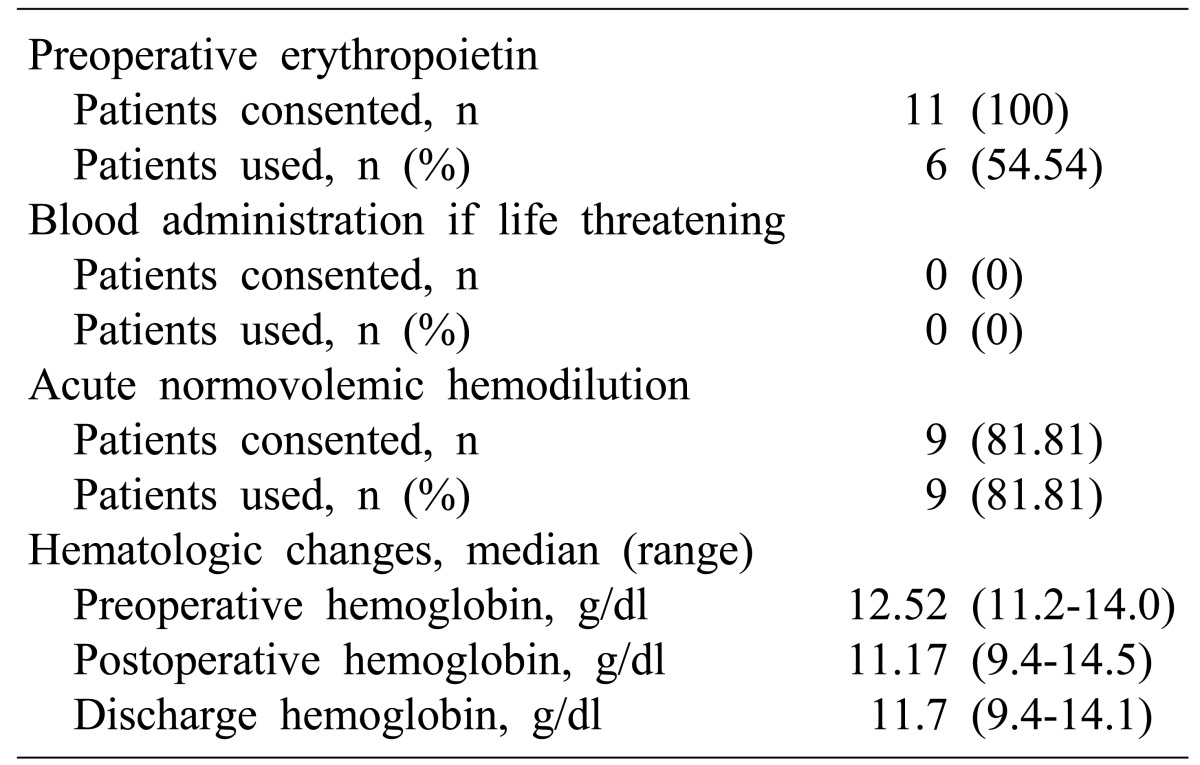
We adopted the major protocols of TF surgery from the existing literature.14 After patients were enrolled in the TF pancreatectomy program, the program team, including physicians, anesthesiologists, and the coordinator discussed with them strategies such as blood augmentation, ANH, and intraoperative cell salvage (ICS). The first two strategies were required therapies for TF pancreatectomy candidates. Enrollment in the TF pancreatectomy program depended on the assessed risk of bleeding, hemostasis status and tolerance of anemia. This was based on history of prior operations, body habitus, age, cardiovascular risk factors, pancreatic fibrosis, pancreatic duct size, presence of other diseases, and overall physiologic status. These many factors were analyzed in combination, but the analysis was subjective due to the absence of any certified scoring/weighting system for these parameters. Medical preparation was aimed at elevating the hemoglobin concentration to normal or above. Recombinant human erythropoietin (rHuEPO) with intravenous (IV) iron sucrose (ferex®, Samyangjenex, Korea, cosmofer Hankookphambio, Korea) were used to elevate hemoglobin levels. Medical preparation with recombinant human erythropoietin and intravenous iron sucrose was done when hemoglobin level was lower then 12.0 g/dl and it was done up to two weeks or as much as possible during other preoperative managements were done.
ANH is an alternative method of transfusion, in which blood is simultaneously removed from a patient and replaced with crystalloid solutions.8 ANH was performed on all the TF pancreatectomy patients. Blood was extracted from the central line and drained without pressure to a citrate-phosphate-dextrose (CPD) bag prior to the start of surgery. Triple lumen central venous catheter was used for central line and Swan Ganz catheter was used when more detailed monitoring for hemodynamic status was required. After the extraction of blood had been completed, the patient's intravascular volume was restored with crystalloid solutions. Sometimes, colloid solutions was used in addition to crystalloid solutions. The patient's hemoglobin concentration was reduced to no lower than an acceptable minimum level based on physiologic status, bleeding risk factors, expected blood loss and medical considerations. Because of the patients' religious beliefs, the ANH blood remained in the operation room without losing connection with the patient for 8 hours, to maintain platelet function. The ANH blood was reinfused at the same rate as the other blood products. A relatively constant amount of blood was extracted for ANH, generally from 2 to 3 units depending on the expected blood loss, preoperative hemoglobin concentration, and the patient's overall physiologic status. Hemodynamic parameters including heart rate, blood pressure, arterial blood gas analysis, and central venous pressure were checked routinely by anesthsiologists during ANH to assess tolerance to the procedure. All the ANH blood which was harvested before the start of the operation was routinely reinfused just before the surgery was completed. ICS was not used throughout the procedure because of the possibility of contamination by cancer cells.
Pylorus-preserving pancreaticoduodenectomy included the entire duodenum, gallbladder, common bile duct (CBD) and pancreas. The duodenum was transected from 2 centimeters distal from the pyloric ring to the end of the duodenum. The proximal cystic duct junction of the CBD and the gallbladder were included in the resection. The pancreas was resected uncinate process, head and neck. When resection margin invasion was suspected, frozen sections of multiple margin regions were also made. An end-to-side duct-to-mucosa type pancreaticojejunostomy (PJ) was performed. In the next stage, an end-to-side choledochojejunostomy (CJ) and side-to-side gastrojejunostomy (GJ) or an end-to-side duodeno-jejunostomy (DJ) was performed. The pancreatic duct was cannulated with an infant-feeding catheter and drained externally through the jejunum. Negative pressure was applied to the feeding tube for effective drainage of pancreatic juice. RAMPS procedure was done as described in detail previously.15 The pancreas was transected at the neck. Then, the celiac lymph node was dissected. After lymph node dissection was complete, the posterior extent of the dissection required was decided on. Dissection was done to the level of the adrenal gland on the anterior and posterior planes. Laparoscopic distal pancreatectomy was performed with five trocars. After a pneumoperitoneum was made, the greater omentum was divided to allow entrance to the lesser sac. The pancreas was mobilized to visualize the proximal splenic artery and the superior mesenteric and splenic trunk. Then a space was identified behind the neck of the pancreas, and the pancreas was transected using that approach. The spleen was resected with the pancreas.
Hemoglobin levels were checked the morning after the operations. Every patients was managed according to a standard pathway. A proton pump inhibitor was given to the patients as prophylactic agent for stress ulcers, and octreotide treatment was not given. Before the end of each operation, drains were inserted aroud the PJ and the CJ. The time of drainage removal was determined according to the amount of the drainge and amylase concentration in the drainage (less than three times the blood amylase concentration and less than 50 ml per day) If the amylase concentration in the drainage remained high after postoperative days 10 the drainge was removed, depending on the clinical findings. The pancreatic duct tube was removed after confirming the absence of pancreatic fistula by checking a ductogram on postoperative day 14.
Postoperative managements in the TF pancreatectomy patients consisted of blood conservation by minimizing blood sampling. Blood augmentation with erythropoietin and iron sucrose was used in selected TF pancreatectomy patients depending on the postoperative hemoglobin level.
Pancreatic fistula was diagnosed if there was more than 10 ml of drainage, and the amylase concentration of the drainage was three or more times the serum amylase concentration after postoperative day 10, following the International Study Group for Pancreatic Fistula (ISGPF) classification.16 Biliary fisula was diagnosed as bilirubin-rich drainage of more than 50 ml per day or after the 10th postoperative day. Postoperative bleeding was defined as a decrease of hemoglobin concentration of more than 2 g/dl at 24 hours after surgery, or relaparotomy for bleeding control.17 Abdominal abscess was defined when abnormal fluid collection was seen on abdominal CT scan, or the drainage was purulent. If nasogastric tube insertion was required after postoperative day 10, delayed gastric emptying was diagnosed. Lung complications were defined as any lung abnormality such as pneumonia, atelectasis, or pleural effusion on postoperative follow-up chest plain films.
Go to : 
Table 1 presents patient demographics, tumor character istics and baseline laboratory data. The mean age of the 11 patients was 62; six patients were female, three had hypertension, one had diabetes mellitus and another had chronic kidney disease. One patient had undergone earlier gastrointestinal surgery.
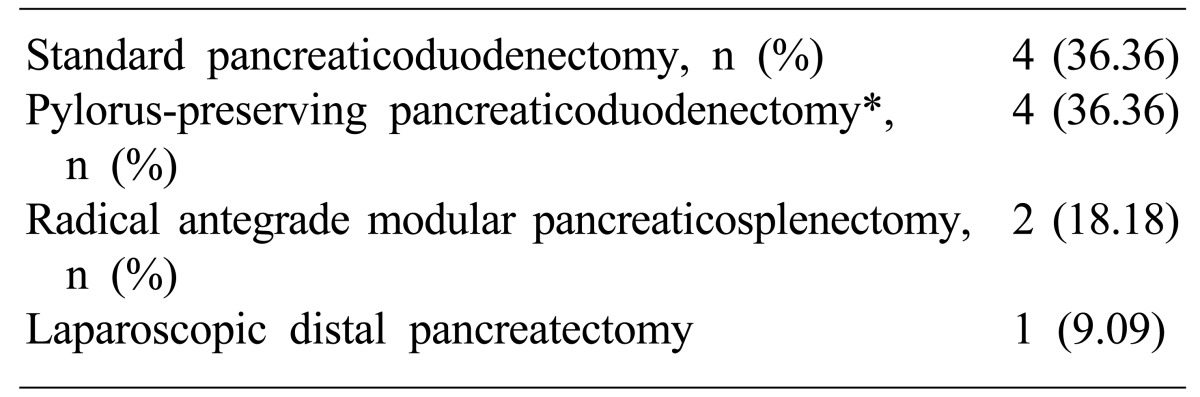
Table 2 shows the types of operation for peripancreatic lesions performed. The curative operations for various diseases in this study were standard pancreaticoduodenectomy in four patients, PPPD in four patients, RAMPS in two patients, and laparoscopic distal pancreatectomy in one patient.
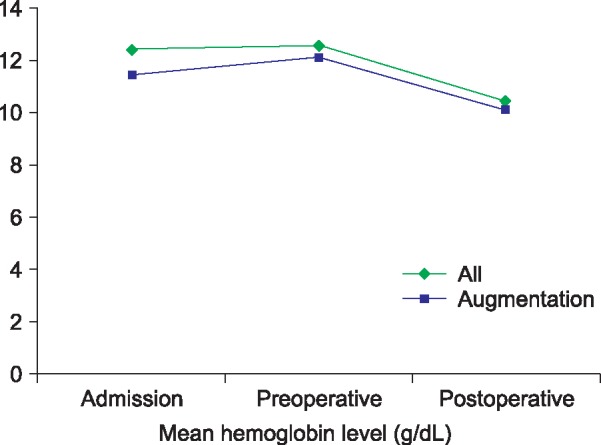
Table 3 and Fig. 1 give details of the perioperative blood management and the changes in hemoglobin level in the individual pancreatectomy patients. The mean preoperative hemoglobin level was 12.6 (11.2-14.0) and the mean postoperative hemoglobin was 10.9 (9.4-14.5). The mean hemoglobin level at discharge was 11.7 (9.4-14.1), and the mean hemoglobin change was –1.35 g/dl (–3.8-+1.2). ANH was carried out in nine patients.
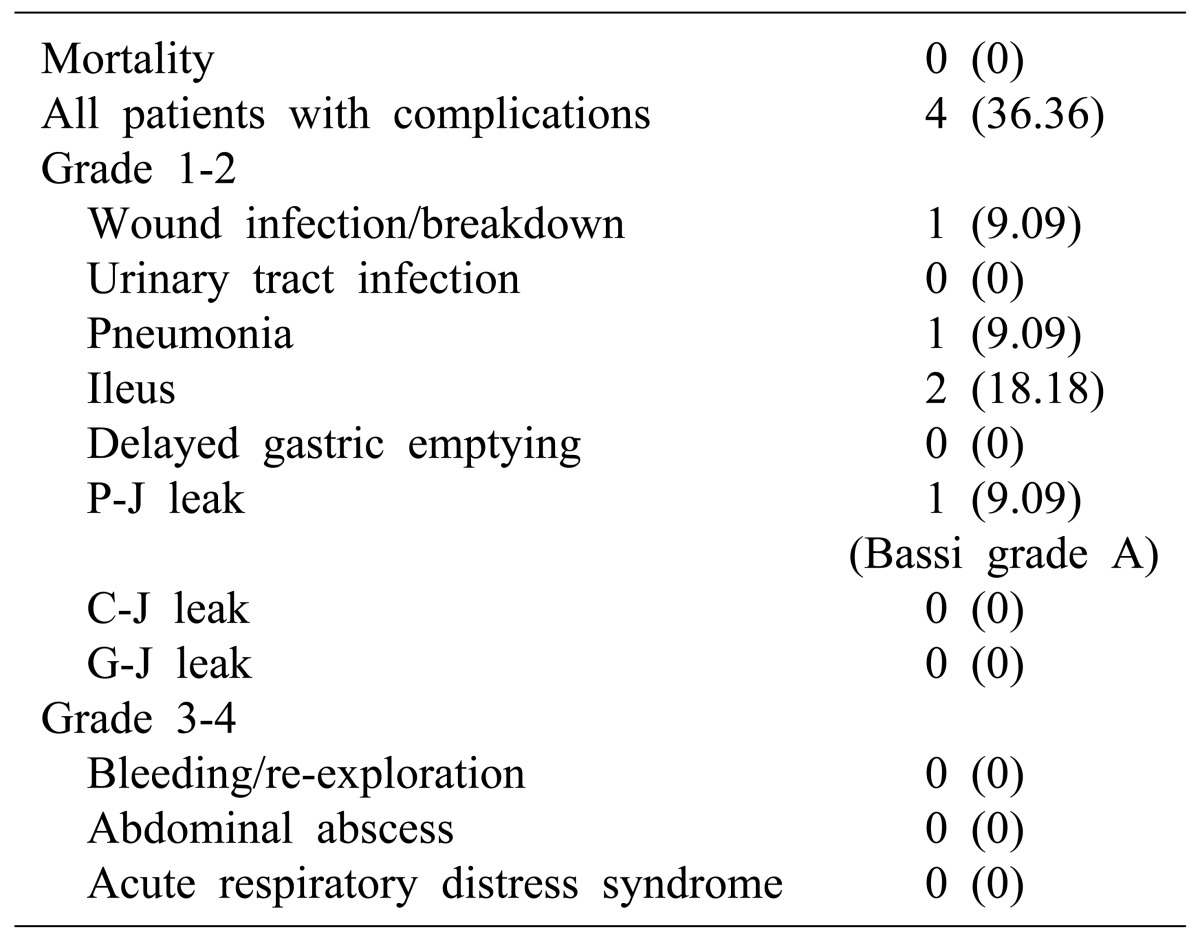
Postoperative complications occurred in 4 patients of the TF pancreatectomy patients (Table 4). One patient suffered wound infection and one pneumonia. Two patients had postoperative ileus. There were no instances of postoperative bleeding requiring blood transfusion or re-exploration. Anastomosis leaks were found in one patient (Bassi grade A pancreaticojejunostomy leak). There were no major bleeding complications bleeding or abdominal abscesses requiring reoperation and no deaths.
Go to : 
Whipple's operation was performed for the first time in 1935.18 Various pancreatectomies have been performed since then as treatments for peripancreatic lesions.1517 Advances in surgical technique and medications have reduced the morbidity and mortality of pancreatectomy and improved surgical outcomes.192021 However blood transfusions are frequently carried out in the perioperative period. A report that the prognosis of patients undergoing pancreas operations with transfusion was worse than that of those that do not receive trasfusion, together with the frequent need for transfusions, has led many patients who do not want transfusions to avoid pancreas surgery.22 To improve this situations, transfusion-free pancreatectomies have been tried recently. TF pancreatectomy has been attempted especially in Jehovah's Witnesses, as well as liver transplantation and liver resection.8 But consecutive TF pancreatectomies have not been attempted but have instead been performed on selected patients individulas.567 On the other hand we performed consecutive TF pancreatectomies and, to the best of our knowledge, this is the first successful consecutive pancreatectomy program involving Jehovah's Witnesses.
In this program, most of the TF pancreatectomy patients received therapy based on three pillars. First, preoperative blood augmentation; IV iron sucrose and erythropoietin were given to patients until their hemoglobin level exceeded 12.0 g/dl. Second, ANH carried out in most of the patients. The decision to do so was made by the anesthesiologist and the amount of ANH was calculated from the basic patients data (expected blood loss, preoperative hemoglobin concentration, and the patient's overall physiologic status as heart rate, blood pressure, and central venous pressure) and depending on the patient's risk of bleeding and general conditions. Third, a transfusion-free program, aimed at minimizing blood loss in the perioperative period.
The mean hemoglobin change after surgery in this trial was 1.35 (–1.2-3.8). Regarding preoperative blood augmentation was performed and hemoglobin level was corrected to 12.0 g/dl or over this range of hemoglobin change was considered tolerable. There were no major complications except Bassi grade A pancreatic fistula, postoperative ileus, wound infection and pneumonia. There were no grade 3 or 4 complications in terms of bleeding, or complications requiring re-exploration. Our results show that the TF pancreatectomy can be carried out safely.
There are some limitations to this study. First, there were no objective selection guidelines for accepting candidates into the TF pancreatectomy program. Such preoperative selection guidelines are needed in future. Second, the number of cases was too small to establish definitively that TF pancreatectomy is safe. Therefore, a large prospective study should be carried out. Third, the duration of the study was insufficient to demonstrate the oncologic effects of TF pancreatectomy in cancer patients.
Although our study has some limitations, it is, to the best of our knowledge, the first successful pancreatectomy program on selected patients in the form of a series of operations without blood transfusion.
In conclusion, if careful preoperative preparation and management is provided by a team approach, TF pancreatecmies can carried out successfully in selected patients without serious complications.
Go to : 
ACKNOWLEDGEMENTS
We thank Yu Jin, Park (Bloodless Center, Soonchunhyang University Hospital) and So Ra Park (Department of Surgery, Soonchunhyang University Hospital) for collecting data.
Go to : 
References
1. Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996; 334:1685–1690. PMID: 8637512.
2. Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. First of two parts--blood transfusion. N Engl J Med. 1999; 340:438–447. PMID: 9971869.
3. Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. Second of two parts--blood conservation. N Engl J Med. 1999; 340:525–533. PMID: 10021474.
4. Sloand EM, Pitt E, Klein HG. Safety of the blood supply. JAMA. 1995; 274:1368–1373. PMID: 7563562.

5. Shiozawa S, Haga S, Kumazawa K, Tsuchiya A, Masuda T, Inose S, et al. Pylorus-preserving pancreaticoduodenectomy without homologous blood transfusion in a Jehovah's Witness with pancreatic cancer: report of a case. Hepatogastroenterology. 2003; 50:272–274. PMID: 12630039.
6. Atabek U, Spence RK, Pello M, Alexander J, Camishion R. Pancreaticoduodenectomy without homologous blood transfusion in an anemic Jehovah's Witness. Arch Surg. 1992; 127:349–351. PMID: 1347993.

7. Magner D, Ouellette JR, Lee JR, Colquhoun S, Lo S, Nissen NN. Pancreaticoduodenectomy after neoadjuvant therapy in a Jehovah's Witness with locally advanced pancreatic cancer: case report and approach to avoid transfusion. Am Surg. 2006; 72:435–437. PMID: 16719200.

8. Jabbour N, Gagandeep S, Mateo R, Sher L, Strum E, Donovan J, et al. Live donor liver transplantation without blood products: strategies developed for Jehovah's Witnesses offer broad application. Ann Surg. 2004; 240:350–357. PMID: 15273561.
9. Nishida S, Madariaga JR, Santiago S, Quintini C, Palaios E, Gyamfi A, et al. Right trisectionectomy of the liver for intrahepatic cholangiocarcinoma with bile duct invasion in a Jehovah's Witness. J Hepatobiliary Pancreat Surg. 2007; 14:312–317. PMID: 17520209.

10. Lucas DJ, Schexneider KI, Weiss M, Wolfgang CL, Frank SM, Hirose K, et al. Trends and risk factors for transfusion in hepatopancreatobiliary surgery. J Gastrointest Surg. 2014; 18:719–728. PMID: 24323432.

11. Konstantinidis IT, Allen PJ, D'Angelica MI, DeMatteo RP, Fischer ME, Grant F, et al. Pancreas and liver resection in Jehovah's Witness patients: feasible and safe. J Am Coll Surg. 2013; 217:1101–1107. PMID: 23880361.

12. Choi SB, Lee JS, Kim WB, Song TJ, Suh SO, Choi SY. Efficacy of the omental roll-up technique in pancreaticojejunostomy as a strategy to prevent pancreatic fistula after pancreaticoduodenectomy. Arch Surg. 2012; 147:145–150. PMID: 22351908.

13. Hwang DW, Han HS, Yoon YS, Cho JY, Kwon Y, Kim JH, et al. Laparoscopic major liver resection in Korea: a multicenter study. J Hepatobiliary Pancreat Sci. 2013; 20:125–130. PMID: 23001191.
14. Choi YY, Seo D, Choi D, Kim JH, Lee KJ, Ok SY. Comparison of blood transfusion free pancreaticoduodenectomy to transfusion-eligible pancreaticoduodenectomy. Am Surg. 2011; 77:81–87. PMID: 21396312.

15. Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. 2003; 133:521–527. PMID: 12773980.

16. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138:8–13. PMID: 16003309.

17. Tran KT, Smeenk HG, van Eijck CH, Kazemier G, Hop WC, Greve JW, et al. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004; 240:738–745. PMID: 15492552.
18. Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of ampulla of vate. Ann Surg. 1935; 102:763–779. PMID: 17856666.
19. Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg. 1987; 206:358–365. PMID: 3632096.

20. Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990; 211:447–458. PMID: 2322039.
21. Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996; 223:273–279. PMID: 8604907.
22. Yeh JJ, Gonen M, Tomlinson JS, Idrees K, Brennan MF, Fong Y. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumour of the pancreas. Br J Surg. 2007; 94:466–472. PMID: 17330243.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download