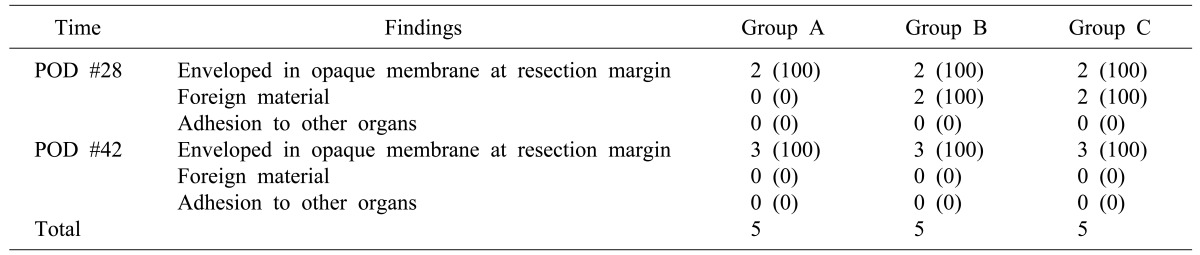
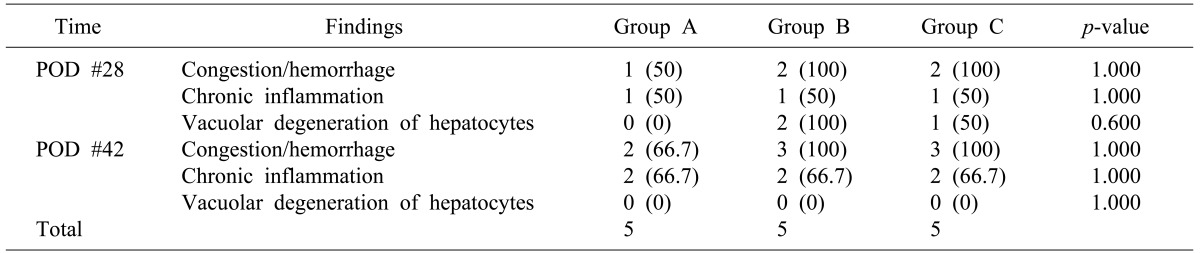
Methods
This study was conducted in accordance with the Korea Food and Drug Administration notification No. 2012-61 'Good Laboratory Practice' (Aug 24, 2012) and Organization for Economic Co-operation and Development Principles of Good Laboratory Practice (1997) in consultation with the sponsor and was approved by our Institutional Animal Care and Use Committee (Approval No. IACU 12-KE-054).
Fifteen mini-pigs (35±5 kg) (Medikinetics mini-pig supplies and services) were randomly allocated into 3 groups: SurgiGuard® (Group C [test], n=5), Surgicel® (Group B [reference], n=5), or none (Group A [control], n=5). Before surgery, all mini-pigs were weighed and blood samples were taken to determine the complete blood cell count (CBC), including white blood cell (WBC), red blood cell (RBC), and platelet (PLT) counts; C-reactive protein (CRP); and liver function tests (LFT), including alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Anesthesia was induced with zoletil and rompun, and the hair in the surgical region (upper abdomen) was removed. Endotracheal intubation was performed and anesthesia was maintained by isoflurane inhalation. The animals were monitored during the procedure by recording the pulse rate and oxygen saturation.
The surgical region was disinfected with povidone iodine and alcohol, followed by the opening of the upper abdominal cavity for liver exposure. Similar to human liver resection, the hepatoduodenal ligament was dissected, and the left lobe was identified for wedge resection. Parenchymal resection of the liver was performed using the Kelly clamp-crushing technique. If the number of bleeding blood vessels was >1, all bleeding blood vessels were closed with forceps except for one blood vessel. However, if the number of bleeding blood vessels was <1, blood vessels were incised to generate one bleeding vessel. Subsequently, ORC of either Surgicel® or SurgiGuard® was applied to the resection margin. The resected liver was weighed.
Blood loss was measured at the time of exposing the resection margin after the confirmation of hemostasis. Blood loss was measured for 5 minutes after the resection. If bleeding was not stopped after 5 minutes, this was regarded as a failure of 1st hemostasis, and blood loss was measured twice at every 2 minutes (i.e., 7 minutes and 9 minutes after resection). The control group received no topical treatment at the resection margin after liver resection. For the 1st hemostasis in the control group, reference group and test group, cotton gauze sheets were applied to the resection margin immediately after resection. Five minutes later, one sheet was applied if 1st hemostasis had not been achieved; this was repeated 2 minutes later if complete hemostasis had not been achieved at the 2nd measurement (at 7 minutes after resection). If complete hemostasis was not achieved after the 3rd measurement (at 9 minutes after resection), a mechanical or thermal method was performed to stop the bleeding.
Detailed measurement of blood loss was performed as follows. In the control group, one sheet of a sterilized waterproof surgical drape was placed below the region of resection, and blood was absorbed by sterilized gauze immediately after the liver resection. In the reference group and test group, two sheets of sterilized waterproof surgical drapes were placed below the region of resection. Surgicel® and SurgiGuard® were applied and one sheet of surgical drapes was removed at the same time. The blood in the surgical field was absorbed by sterilized gauze. The wet gauzes were weighed, and the blood loss was calculated as the difference between the weight of the wet gauze and the premeasured weight of the dry gauze (
Fig. 2). After measurement of the blood loss, the surgical region was arranged to avoid adhesion with the resection margin of the liver. The muscle and skin were then sutured. The time of every procedure of the operation was recorded.
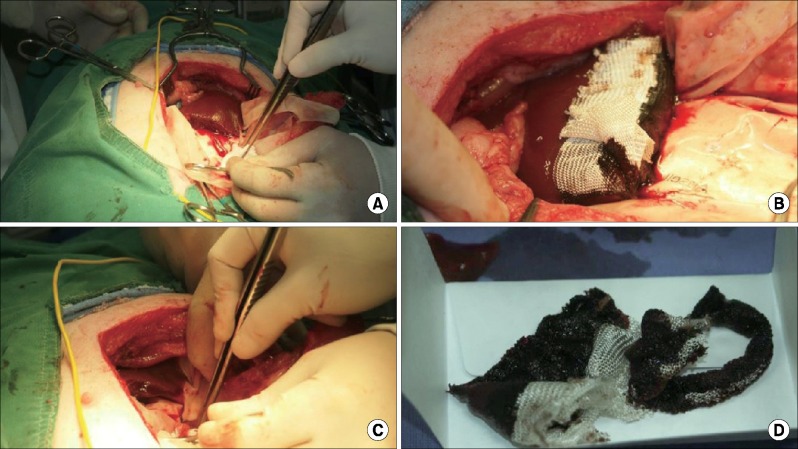 | Fig. 2Experimental procedures: (A) After wedge resection, (B) Application of hemostatic material, (C) Blood absorption by sterilized gauze and (D) Measurement of blood loss.
|
After surgery, the animals were permitted food and water as normal upon recovery from anesthesia. They were subsequently monitored once daily. If any abnormality was found, the type and date of occurrence and severity of signs were recorded for each abnormality. All animals were weighed once weekly throughout the experimental period. One week after the operation, a blood sample was taken to measure the same parameters determined preoperatively.
The animals in each study group (control, reference, and test) were randomly divided into 2 subgroups for necropsy. Two mini-pigs were allocated to 1st necropsy group and the other 3 mini-pigs were allocated to 2nd necropsy group. Necropsy was performed 4 weeks after the operation in the 1st necropsy group and 6 weeks after surgery in 2nd necropsy group. All mini-pigs were fasted before their necropsy for at least 12 hours. Before anesthesia, another set of blood samples was obtained from each mini-pig. The animals were deeply anesthetized with zoletil and rompun and euthanized by exsanguination from the carotid artery. The resected livers were examined grossly. After recording the results, the livers, including resection margins, were fixed in 10% neutral formalin solution. They were then embedded in paraffin, and microsections with a thickness of 4-5 µm were made from the blocks. Hematoxylin & Eosin - stained slides were prepared, and the specimens were examined with an optical microscope.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download