This article has been
cited by other articles in ScienceCentral.
Abstract
We present a rare case of functional stenosis of the jejunal loop following left hepatectomy and hepaticojejunostomy long after pylorus-preserving pancreaticoduodenectomy (PPPD), which was successfully managed by balloon dilation. A 70-year-old Korean man had undergone PPPD 6 years before due to 1.8 cm-sized distal bile duct cancer. Sudden onset of obstructive jaundice led to diagnosis of recurrent bile duct cancer mimicking perihilar cholangiocarcinoma of type IIIb. After left portal vein embolization, the patient underwent resection of the left liver and caudate lobe and remnant extrahepatic bile duct. The pre-existing jejunal loop and choledochojejunostomy site were used again for new hepaticojejunostomy. The patient recovered uneventfully, but clamping of the percutaneous transhepatic biliary drainage (PTBD) tube resulted in cholangitis. Biliary imaging studies revealed that biliary passage into the afferent jejunal limb was significantly impaired. We performed balloon dilation of the afferent jejunal loop by using a 20 mm-wide balloon. Follow-up hepatobiliary scintigraphy showed gradual improvement in biliary excretion and the PTBD tube was removed at 1 month after balloon dilation. This very unusual condition was regarded as disuse atrophy of the jejunal loop, which was successfully managed by balloon dilation and intraluminal keeping of a large-bore PTBD tube for 1 month.
Go to :

Keywords: Balloon dilation, Functional stenosis, Disuse atrophy, Hepaticojejunostomy, Redo surgery
INTRODUCTION
Repeated major hepatobiliary surgery can carry unusual unexpected complications, which may not happen at the first-time surgery.
123 The jejunal loop limb is the primary resource for biliary and pancreatic reconstruction.
4 After Roux-en-Y hepaticojejunostomy or classical reconstruction following pancreaticoduodenectomy, only clear bile and/or pancreatic juice passes the jejunal loop limb, thus it works as a narrow-caliber conduit and can suffer disuse atrophy long time later due to lack of food passage. However, such a jejunal loop limb can be used again for repeated major hepatobiliary surgery without any significantly serious problem because it will be well accommodated at different surgical settings.
We present a rare case of functional stenosis of the jejunal loop following left hepatectomy and hepaticojejunostomy long after pylorus-preserving pancreaticoduodenectomy (PPPD), which was successfully managed by balloon dilation.
Go to :

CASE
A 70-year-old Korean man had undergone PPPD 6 years before due to 1.8 cm-sized well differentiated adenocarcinoma of the distal bile duct (T3N0M0 lesion) (
Fig. 1A). One month before admission, he suffered from obstructive jaundice and workup led to the diagnosis of recurrent bile duct cancer mimicking perihilar cholangiocarcinoma of type IIIb (
Fig. 1B). Cholangioscopic biopsy through the percutaneous transhepatic biliary drainage (PTBD) tract showed the presence of atypical cell clusters in the ulcer debris (
Fig. 1C, D), suggestive of adenocarcinoma. Thus late tumor recurrence was highly suspected. Thus we decided to perform resection of the left liver and caudate lobe as well as resection of the residual bile duct with a curative intent.
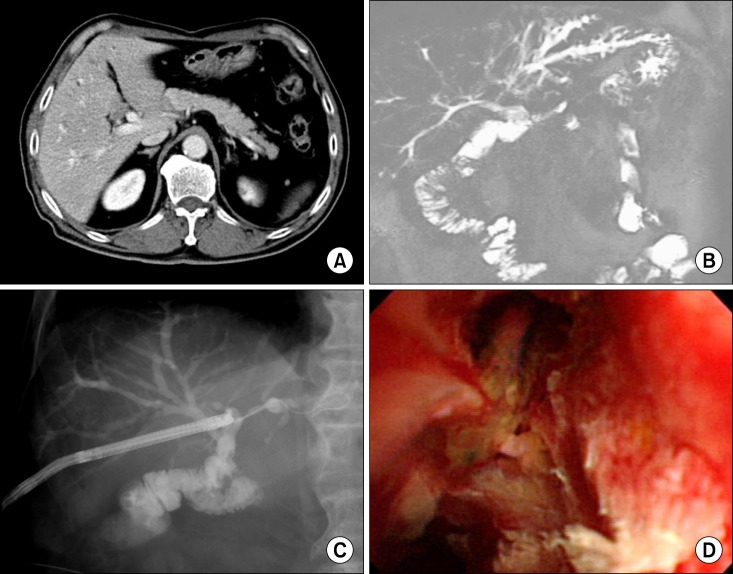 | Fig. 1The preoperative assessment process. (A) The postoperative status following pylorus-preserving pancreaticoduodenectomy (PPPD) was visible on the computed tomography (CT) scan; (B) Recurrent bile duct cancer mimicked perihilar cholangiocarcinoma of type IIIb on magnetic resonance cholangiography; and (C) and (D) Cholangioscopic biopsy was attempted for tissue confirmation.
|
Since the left liver appeared to be 45% of the total liver volume (
Fig. 2A) and he underwent PPPD, we performed left portal vein embolization (PVE) to facilitate left liver resection as well as to ensure patient safety. Follow-up computed tomography (CT) 10 days after PVE showed a noticeable atrophy of the left liver (40% of the total liver volume:
Fig. 2B), thus we performed resection of the left liver and caudate lobe and remnant extrahepatic bile duct at 13 days after left PVE (
Fig. 3). There were heavy adhesions around the left liver and caudate lobe, thus very meticulous time-consuming dissection was necessary. The pre-existing choledochojejunostomy from PPPD was transected and a frozen-section biopsy revealed that there was no tumor invasion at this area. Thus we decided to use the jejunal loop for biliary reconstruction instead of making a new Roux-en-Y jejunal loop limb. This finding might implicate de novo malignancy or skipped bile duct cancer recurrence because the pre-existing choledochojejunostomy was spared from tumor invasion. Removal of the liver and residual extrahepatic bile duct resulted in two separate bile duct openings at the remnant right liver, which were unified by customized unification sutures with 5-0 and 6-0 Prolenes. The opening at the jejunal loop, which was correspondent to the prior choledochojejunostomy, was used for biliary reconstruction. To match the jejunal limb length, the retrocolic tunnel portion was extensively mobilized. The patient had a PTBD catheter at the time of surgery, thus this catheter was replaced with a 3 mm-sized silatic tube and its tip with multiple side-holes were placed within the jejunal loop across the newly formed hepaticojejunostomy (
Fig. 2C).
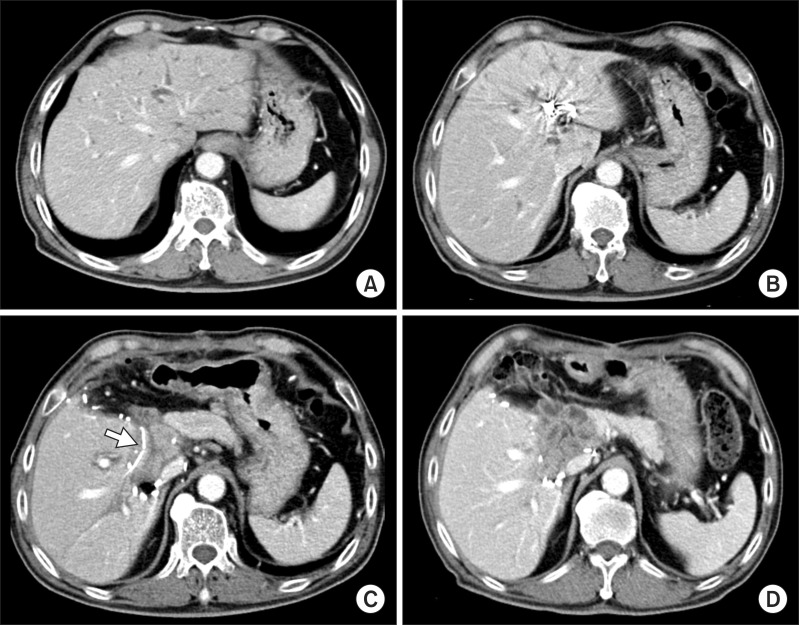 | Fig. 2Sequences of CT scan follow-up. (A) Left liver and caudate lobe occupied 45% of the total liver volume; (B) Follow-up CT scan 10 days after PVE showed a noticeable atrophy of the left liver; (C) Follow-up CT scan 5 days after left liver resection showed deeply seated location of the hepaticojejunostomy and intraluminal location of a tube (arrow); and (D) Postoperative 2 month CT scan showed uneventful recovery from the redo surgery.
|
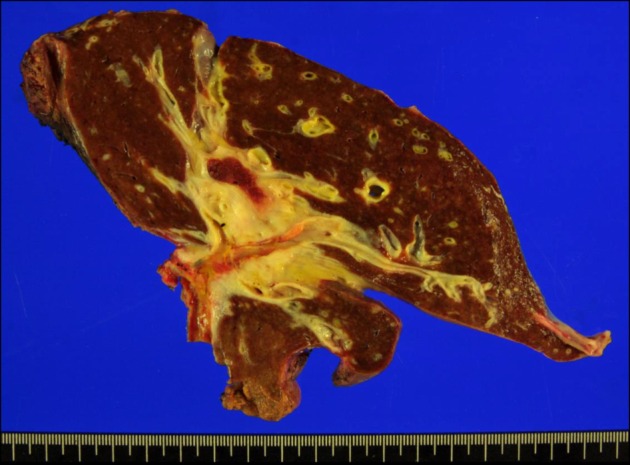 | Fig. 3Gross photograph of the resected liver specimen. A wide area of the hepatic hilum was involved with extension beyond the bile duct, thus the whole paracaval portion had to be removed.
|
The patient recovered uneventfully without any surgical complication. At 14 days after surgery, the replaced PTBD tube was test-clamped for removal. On the day after test-clamping, cholangitis happened unexpectedly. Hepatobiliary scintigraphy showed a 16% excretion rate at 90 minutes, implicating significant outflow disturbance (
Fig. 4A). Direct cholangiography through the PTBD tube showed uneventful filling of the intrahepatic duct and hepaticojejunostomy, but a passage into the jejunal limb was significantly impaired (
Fig. 5A, B).
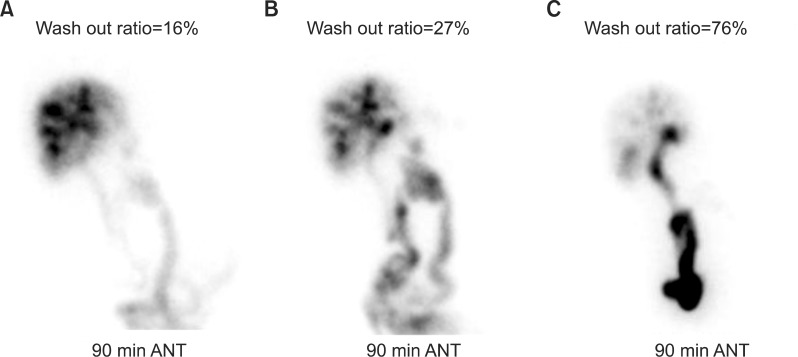 | Fig. 4Sequences of hepatobiliary scintigraphy follow-ups taken at postoperative 2 weeks (A), 1 week after balloon dilation (B) and 4 weeks after balloon dilation (C).
|
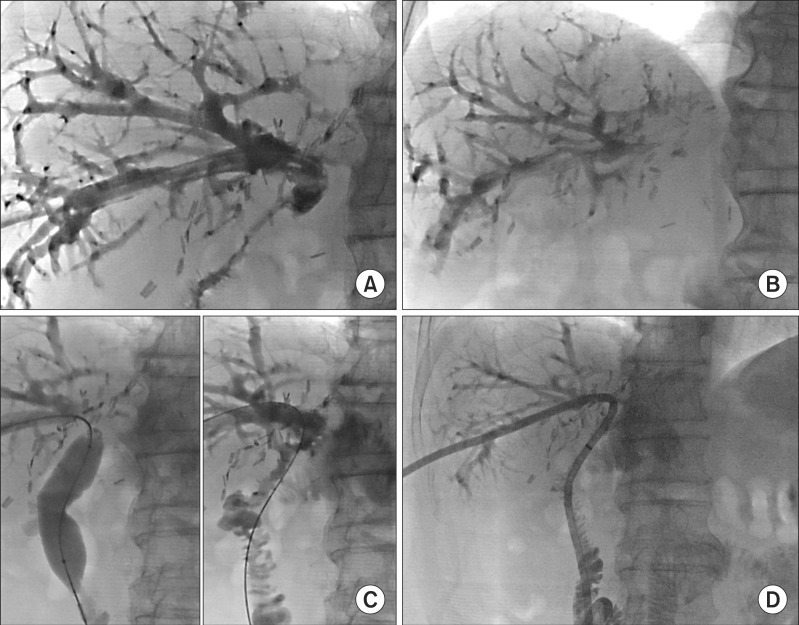 | Fig. 5Sequences of tube cholangiography. (A) and (B) Direct cholangiogram through the PTBD tube showed uneventful filling of the intrahepatic duct and hepaticojejunostomy, but outflow passage at 5 minutes was impaired; and (C) and (D) Balloon dilation of the afferent jejunal loop was performed by using a 20 mm- wide balloon and a large-bore catheter was placed deep into the afferent jejunal loop.
|
With the integration of the clinical information and radiologic imaging findings, we presumed that there was a functional outflow stenosis probably due to disuse atrophy of the afferent jejunal loop. We performed balloon dilation of the afferent jejunal loop by using a 20 mm-wide balloon (
Fig. 5C, D). A 1-week follow-up hepatobiliary scintigraphy showed a 27% excretion rate at 90 minutes (
Fig. 4B), implicating insufficient improvement.
To ensure patient security, the patient was discharge with clamped PTBD tube. Three weeks later, follow-up hepatobiliary scintigraphy showed a 76% excretion rate at 90 minutes (
Fig. 4C). Thereafter, the PTBD tube was removed. The patient is doing well with normal liver function and no evidence of tumor recurrence at 6 months after the second surgery (
Fig. 2D).
Go to :

DISCUSSION
Functional stenosis of the afferent jejunal loop from disuse atrophy would be an imaginary concept, in which its presence was not fully demonstrated yet.
56 Since we have performed redo surgery using the pre-existing afferent jejunal loop limb for more than 20 times, we have never experienced it before. Since this was the first case indicating a functional stenosis of the afferent jejunal loop, we intended to assess its underlying pathogenesis through all available imaging study modalities including direct cholangiography and hepatobiliary scintigraphy. Through these studies, we reached to the conclusion that the hepaticojejunostomy was patent but afferent jejunal loop did not work properly thus causing functional stenosis. During the interventional procedure of jejunal loop balloon dilation, we found that the initial diameter of the afferent jejunal loop was small, but it was easily expanded by balloon dilation. Only after jejunal loop balloon dilation, did it work as a fully functioning biliary-pancreatic conduit. These clinical sequences implicated that there was a disuse atrophy existing at the afferent jejunal loop 6 years after PPPD. This type of jejunal loop disuse atrophy has not been reported before, but it is reasonable to forecast its possibility.
Repeated major hepatobiliary surgery can carry various unusual, unexpected complications, thus every available preventive option should be considered.
123 First of all, in this patient, the left PVE was intentionally introduced because impairment of liver function after left liver resection and heavy adhesion around the previous operation site was anticipated.
7 Artificial induction of the left liver atrophy would make the mobilization the left liver and hepatic hilum easier. Right PVE usually intends to induce left liver regeneration, but left PVE are more readily applicable to induce left liver atrophy. We have performed left PVE for highly selected patients with high operative risk. As the second option, we placed the PTBD tube deep into the jejunal loop passing through the hepaticojejunostomy. The tip of the original PTBD tube was located within the intrahepatic duct, thus it may not be optimized for biliary decompression to prevent anastomotic leak. The third option was very meticulous dissection of the pre-existing choledochojejunostomy from PPPD. Any irrelevant dissection can make damage to the jejunal loop, by which we can encounter a difficult situation of not using it again even in the absence of tumor invasion at the jejunal loop. Meticulous bowel loop dissection and absence of tumor invasion enabled us to use the jejunal loop again, which might alleviate the surgical burden definitely.
In conclusion, we present a rare case of functional stenosis of the jejunal loop following left hepatectomy and hepaticojejunostomy long after PPPD, which might be associated with disuse atrophy of the jejunal loop limb. This very unusual condition was successfully managed by balloon dilation and intraluminal keeping of a large-bore PTBD tube for 1 month.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download