This article has been
cited by other articles in ScienceCentral.
Abstract
Endovascular stenting is accepted as an effective treatment for patients with Budd-Chiari syndrome (BCS). We herein present a case of successful endovascular treatment. A 46-year-old woman, who was followed up for 10 years after a diagnosis of BCS, showed progression progressive of liver cirrhosis and deterioration deteriorated of liver function. Three main hepatic veins were thrombosed with complete occlusion of the suprahepatic of the inferior vena cava (IVC); thus, hepatic venous blood flow was draining into the inferior right hepatic veins through the intrahepatic collaterals and passed passing through the subcutaneous venous collaterals. She underwent endovascular stenting of the IVC for palliation. A septoplasty needle was passed through the occluded IVC through into the internal jugular vein access and then to access the femoral vein using a snare wire. Severe elastic recoiling was observed after balloon dilatation; thus, a 28×80 mm stenting was done inserted across the occlusion, and repeat double ballooning was performed. The final venogram shows showed restored IVC inflow. The patient began to lose body weight 1 day after stenting, and edema disappeared within 1 week. She is was doing well at the 6 month follow-up visit with nearly normal liver function and marked resolution of cutaneous venous engorgement. In conclusion, endovascular stenting appeared to be an effective treatment to alleviate portal pressure and to prevent BCS-associated complications; thus, endovascular stenting should be considered before marked hepatic vein stenosis or complete occlusion occurs in patients with BCS.
Go to :

Keywords: Budd-Chiari syndrome, Stenting, Hepatic vein, Inferior vena cava, Hepatic vein
INTRODUCTION
Budd-Chiari syndrome (BCS) is a hepatic venous outflow obstruction at any level from the small hepatic veins to the junction of the inferior vena cava (IVC) and the right atrium. The cause of BCS is not completely understood. Hepatic vein or IVC lesions include membranous or stenotic obstruction or thrombosis. Thrombosis of the hepatic veins is the most common cause of BCS in Western countries, whereas membranous obstruction of the IVC or the hepatic veins is more common in Eastern countries.
12 Patients with hepatic vein occlusion may present with symptoms and signs of portal hypertension, including ascites, hepatomegaly, splenomegaly, and progressive liver dysfunction.
3
As most of the significant symptoms and signs in patients with BCS are associated with portal hypertension, treatment is usually intended to relieve portal hypertension. The anatomy of the hepatic veins is complex and distorted during progression of BCS; thus, it is not practical to surgically reconstruct the veins. Portal diversion treatment, such as side-to-side portocaval, mesocaval, and mesoatrial shunts, have been used, but these surgical methods are also associated with many serious complications.
Along with advances in radiological intervention technology, endovascular treatment has become a reliable treatment option for patients with symptomatic BCS. Recanalization of occluded major hepatic veins or the IVC facilitates hepatic venous outflow drainage; thus, hepatic congestion is relieved and portal pressure decreases, which is beneficial for recovery of liver function.
45 Endovascular stenting is currently accepted as an alternative effective treatment for BCS when liver transplantation (LT) is unavailable. We herein present a case of successful endovascular treatment performed in a patient with gradually deteriorating BCS.
Go to :

CASE
A 46-year-old woman was followed up for 10 years after a BCS diagnosis (
Fig. 1). She was referred to us for LT due to progressive liver cirrhosis and deteriorated liver function. As a liver donor was unavailable, the patient underwent endovascular stenting of the IVC for palliation.
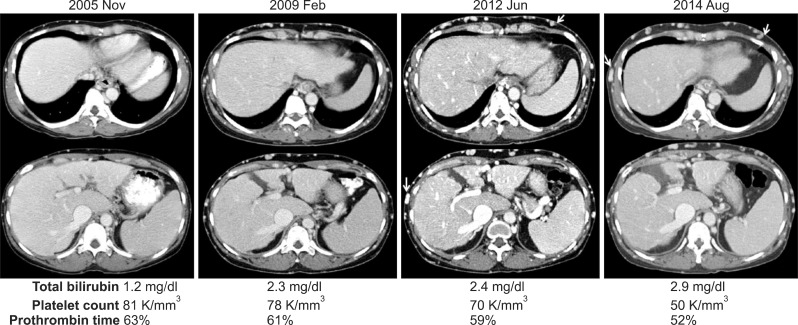 | Fig. 1Clinical sequence in a patient with Budd-Chiari syndrome and a 10 year follow-up. Computed tomography image shows progressive advanced liver cirrhosis with prominent development of subcutaneous venous collaterals (arrows). Liver function and coagulation profiles deteriorated concurrently over time.
|
An abdominal computed tomography (CT) scan showed hepatomegaly and ascites, and gastrointestinal endoscopy revealed esophageal varices. Three main hepatic veins were thrombosed with complete occlusion of the suprahepatic IVC; thus, hepatic venous blood flow was draining into the inferior right hepatic vein through the intrahepatic collaterals and passing through the subcutaneous venous collaterals (
Fig. 2A). She was placed on the LT waiting list due to progressive liver cirrhosis and deteriorating liver function, but there was little possibility of deceased organ allocation considering the preserved status of liver function, and no living donor was available.
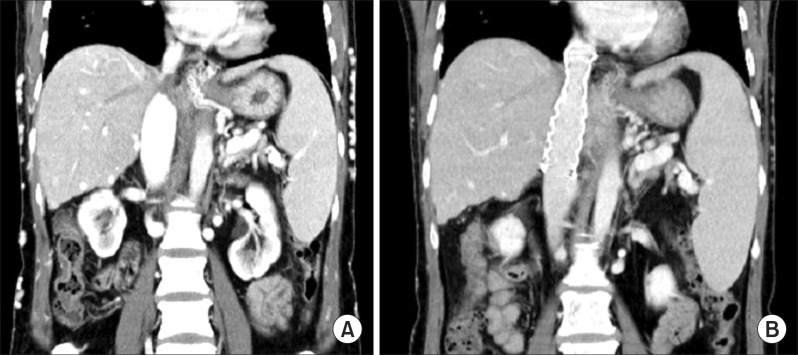 | Fig. 2Computed tomography images revealing the status of the retrohepatic inferior vena cava before (A) and after endovascular stenting (B).
|
She underwent endovascular stenting of the IVC for palliation, rather than LT. A septoplasty needle was passed through the occluded IVC into the internal jugular vein and femoral vein using a snare wire. Severe elastic recoil (>90%) was observed after balloon dilatation; thus, a 28×80-mm stent was inserted across the occluded area, and repeat double ballooning was performed. The final venogram shows restored IVC inflow (
Fig. 3).
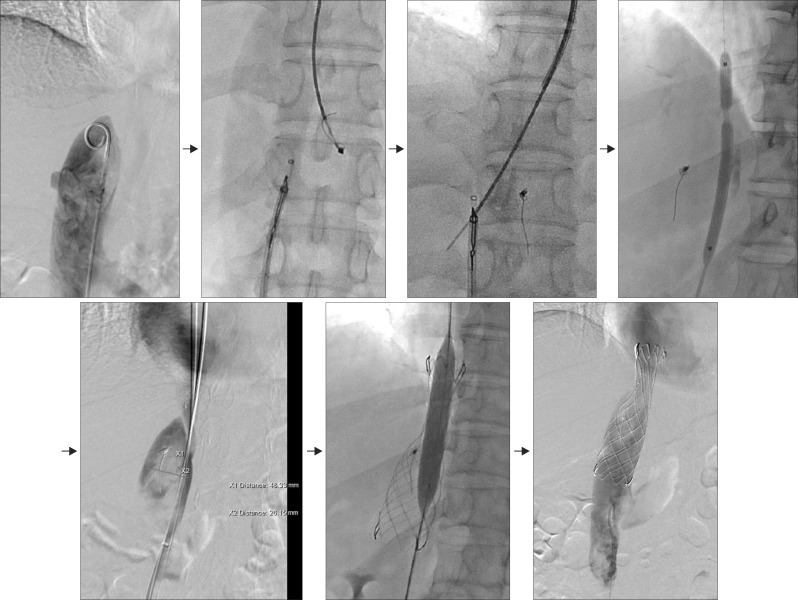 | Fig. 3Interventional endovascular stenting procedure for the inferior vena cava. The rendezvous technique was applied by assessing the internal jugular vein and femoral vein. A 28×80-mm sized stent was placed, and repeat double ballooning was performed.
|
She began to lose body weight 1 day after stenting, and the edema disappeared within the first week. A follow-up CT scan showed that the stented IVC was fully patent (
Fig. 2B) with complete thrombosis of the three main hepatic veins (
Fig. 4A), but intrahepatic collateral flow was draining through the right inferior hepatic vein with spontaneous closure of subcutaneous collateral veins (
Fig. 4B).
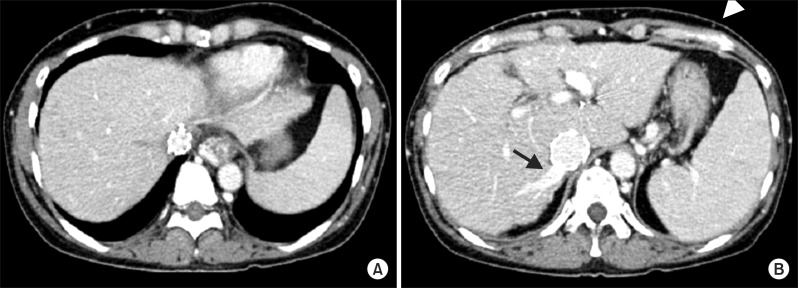 | Fig. 4Follow-up computed tomography image at 3 months shows complete thrombosis of the three main hepatic veins (A), intrahepatic collateral flow drainage through the right inferior hepatic vein (arrow), and collapsed subcutaneous collateral veins (arrow head) (B).
|
Liver function recovered slowly over several weeks. Anticoagulation (warfarin) and anti-platelet therapy (aspirin) was started 4 days after stenting to minimize the risk of thrombotic occlusion of the IVC stent. She was doing well at the 6-month follow-up visit, with nearly normal liver function (total bilirubin, 0.9 mg/dl and platelet count, 77,000/mm3) and marked resolution of subcutaneous venous engorgement.
Go to :

DISCUSSION
Two underlying causes lead to a manifestation of BCS. One is hepatic vein thrombosis, which is often associated with various coagulation defects and disorders.
67 The other is membranous obstruction of hepatic veins or the IVC.
6 This latter cause is more common in Eastern countries and may be congenital in origin. BCS is regarded as a very rare disease entity in Korea.
Restoring hepatic venous outflow is the primary objective when treating BCS. Endovascular treatment is the main therapeutic choice with advances in interventional technology.
48 A transjugular intrahepatic portosystemic shunt (TIPS) is currently the most common intervention for patients with BCS in Western countries because obstruction of the hepatic veins is usually extensive.
89 A TIPS often effectively relieves portal pressure in patients with liver cirrhosis as well as those with BCS, but its indication is limited, particularly in patients with advanced liver cirrhosis. Balloon dilatation or stent insertion can be attempted to recanalize the hepatic venous outflow pathways, when the lumen of a hepatic vein or the IVC is partially maintained.
It is important to pass the occluded area successfully when performing an endovascular intervention because this area may already be completely obliterated. In this present case, the IVC segment around the drainage for the main hepatic vein was completely occluded; thus, a rendezvous technique was applied after passing from internal jugular vein access and capturing the wire from femoral vein access. Conventional balloon dilatation and endovascular stenting follow after the occluded portion is cannulated. Interventional treatment is a safe and effective way to treat BCS.
10
Hepatic venous pressure can drop 25-50% after successful endovascular stenting in patients with BCS, and the 5-year survival rate in patients with mild disease and preserved liver function can be as high as 100%. Survival rates for patients with progressing BCS are about 85% (65-100%) at 1 year and 77% (53-100%) at 5 years.
1112
The common complications of endovascular treatment for BCS include hepatic encephalopathy, bleeding into the abdominal cavity or biliary tract bleeding, and hepatic artery injury, among others. The reported overall incidence of complications is <3%, and mortality rate is about 1%.
13 The incidences of restenosis after balloon expansion and stenting of hepatic veins are 10-20% and 10%, respectively.
11 As the IVC and major hepatic veins are high-flow medium-velocity vessels, anticoagulation therapy is not indicated when using a wire-wall stent. Newly formed endothelium will cover the thrombogenic surface of the stent material within 2-3 weeks. The primary reason why we administered anticoagulant and antiplatelet agents was due to the risk of restenosis because mechanical factors are the most critical for thrombogenesis based on hemodynamics principles.
Collaterals commonly develop in patients with BCS, and these collaterals play an important role in the clinical manifestation of BCS. The rationale of "early diagnosis and early treatment" is not suitable for all patients with BCS. Satisfactory survival can be achieved in patients who are completely compensated by rich collaterals without invasive treatment. Nevertheless, a beneficial treatment procedure should be performed if the patient's conditions worsens at follow-up visits.
14
In conclusion, endovascular stenting appears to be an effective treatment to alleviate portal pressure and prevent BCS-associated complications; thus, endovascular stenting should be considered before marked stenosis or complete occlusion occurs in patients with BCS.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download