This article has been
cited by other articles in ScienceCentral.
Abstract
Left-sided gallbladder (LSGB) is a rare anomaly, but it is often associated with multiple combined variations of the liver anatomy. We present the case of a patient with LSGB who underwent successful resection of perihilar cholangiocarcinoma. The patient was a 67-year-old male who presented with upper abdominal pain and obstructive jaundice. Initial imaging studies led to the diagnosis of Bismuth-Corlette type IIIB perihilar cholangiocarcinoma. Due to the unique location of the gallbladder and combined multiple hepatic anomalies, LSGB was highly suspected. During surgery after hilar dissection, we recognized that the tumor was located at the imaginary hilar bile duct bifurcation, but its actual location was corresponding to the biliary confluence of the left median and lateral sections. The extent of resection included extended left lateral sectionectomy, caudate lobe resection, and bile duct resection. Since some of the umbilical portion of the portal vein was invaded, it was resected and repaired with a portal vein branch patch. Due to anatomical variation of the biliary system, only one right-sided duct was reconstructed. The patient recovered uneventfully without any complication. LSGB should be recognized as a constellation of multiple hepatic anomalies, and therefore, thorough investigations are necessary to enable the performance of safe hepatic and biliary resections.
Go to :

Keywords: Hepatectomy, Perihilar cholangiocarcinoma, Left-sided gallbladder, Anatomical variation
INTRODUCTION
Left-sided gallbladder (LSGB) usually means that the gallbladder is located to the left side of the round ligament without situs inversus viscerum. In addition, the middle hepatic vein should run to the right of the gallbladder and the round ligament itself should originate from the left portal vein to meet its definition. The incidence of LSGB in Korean population is unknown, but its incidence in patients undergoing hepatobiliary surgery at our institution is estimated to be less than 0.1%.
1 The presence of LSGB is often associated with multiple combined anomalies of the portal vein, hepatic artery, and bile duct. Such a constellation of combined hepatic anomalies can make resection of hepatobiliary malignancy difficult or impossible; therefore, it is essential to establish a special operative plan after thorough evaluation of both combined anomaly and tumor extent.
2
In review of the English literature, only two Japanese patients with LSGB have been reported to undergo curative surgery for perihilar cholangiocarcinoma.
2 Here, we present the case of a patient with LSGB who underwent successful resection of perihilar cholangiocarcinoma.
Go to :

CASE
This case was of a 67-year-old male patient who presented with upper abdominal pain and obstructive jaundice. Laboratory examination on admission showed serum total bilirubin level of 9.6 mg/dl. Abdominal computed tomography (CT) and magnetic resonance imaging revealed Bismuth-Corlette type IIIB perihilar cholangiocarcinoma with left portal vein and left hepatic artery invasion, type III portal vein variation, dilated right intrahepatic bile duct, and left liver lobe atrophy (
Fig. 1). Due to the unique location of the gallbladder and combined multiple hepatic anomalies, presence of LSGB was highly suspected (
Fig. 2). The branching pattern of the portal vein appeared strange compared with the usual type III portal vein variation, in which the liver appeared to be divided into 3 parts; the right posterior section, the right anterior section integrated with the left medial section, and the left lateral section (
Fig. 3). For biliary decompression, endoscopic nasobiliary drainage was performed following endoscopic retrograde cholangiography. Surgery was performed when the serum total bilirubin level was 1.7 mg/dl.
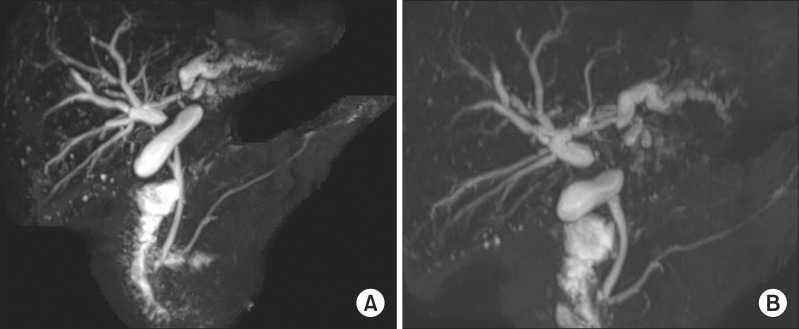 | Fig. 1Magnetic resonance cholangiopancreatography images showing wide separation of the hilar bile ducts (A) without visualization of the usual B4 duct (B).
|
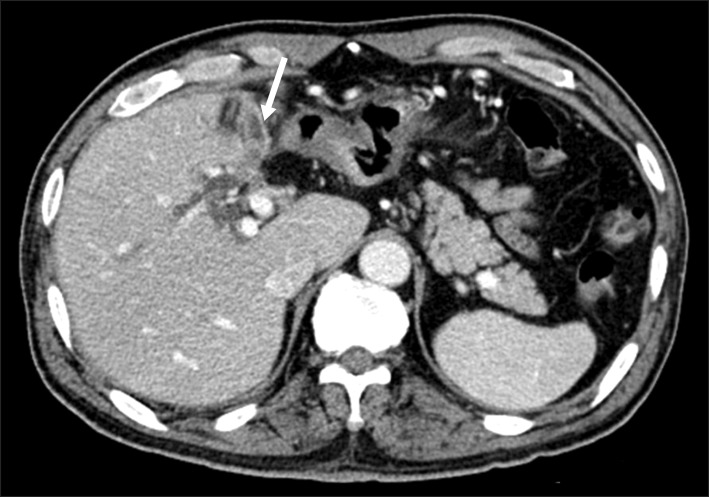 | Fig. 2Computed tomography image indicating the possible presence of the left-sided gallbladder (arrow).
|
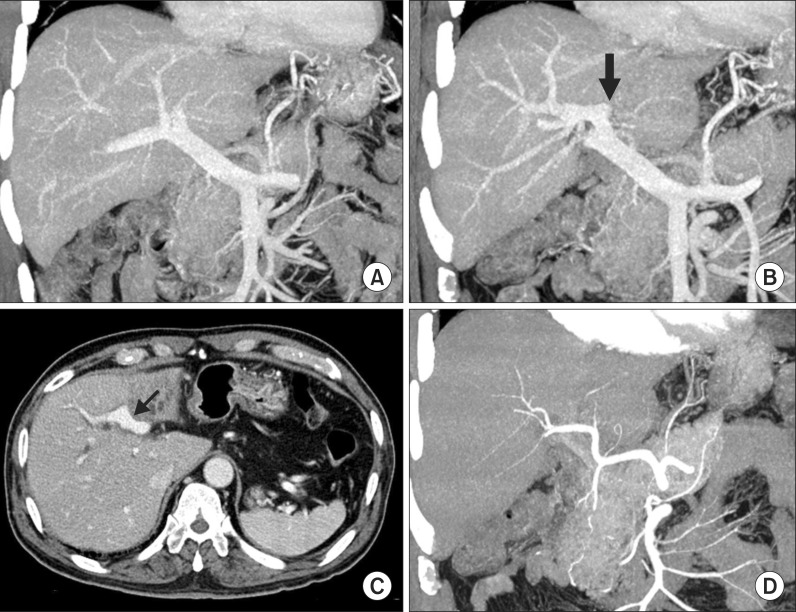 | Fig. 3Computed tomography images showing the hepatic inflow vessels. Portal vein trifurcation is visible with complete occlusion of the left portal vein (A); A black arrow indicates the insertion site of the round ligament (B); The umbilical portion of the portal vein is partially invaded by the tumor (arrow, C); The hepatic arteries do not appear to have significant variation (D).
|
After dissection of the hilar structures, we recognized that the tumor was located at the imaginary hilar bile duct bifurcation, but its actual location was corresponding to the biliary confluence of the left median and lateral sections. After transecting the distal bile duct at the level of the pancreas, we found that there was no vascular invasion of the right-sided liver. Thus, the extent of liver resection included extended left lateral sectionectomy (
Figs. 4,
5). Through the anterior transection approach without the hanging maneuver, the liver was transected and the bile duct was resected en bloc. The right-sided bile duct orifice in the liver was 8 mm in size and single, and probing exploration revealed that it was the common-channel bile duct confluence of the above-mentioned three sections in the right-sided liver. The resection margin was tumor-negative on frozen-section biopsy. Since only the left-sided wall of the umbilical portion of the portal vein was partially invaded by the tumor, the involved portion was excised and then covered with a remnant branch patch after excision of a small adjacent portal vein branch in the left medial section (
Fig. 5).
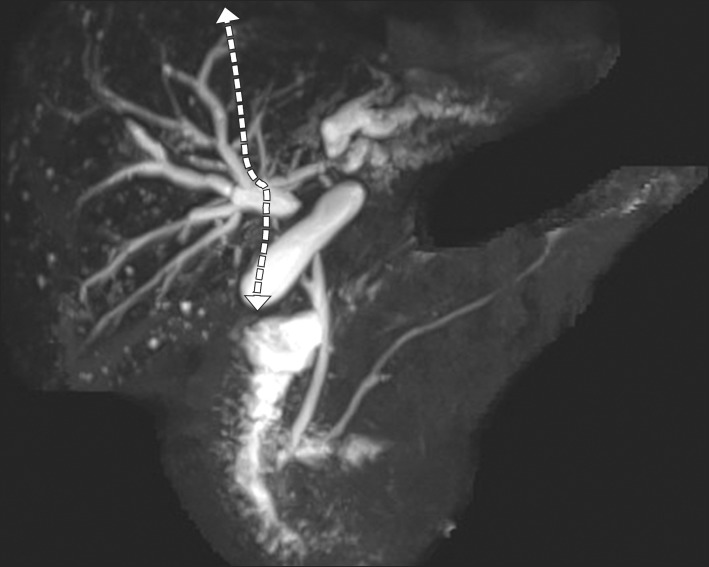 | Fig. 4Magnetic resonance cholangiopancreatography image superimposed by the pre-planned hepatic transection plane (dotted line).
|
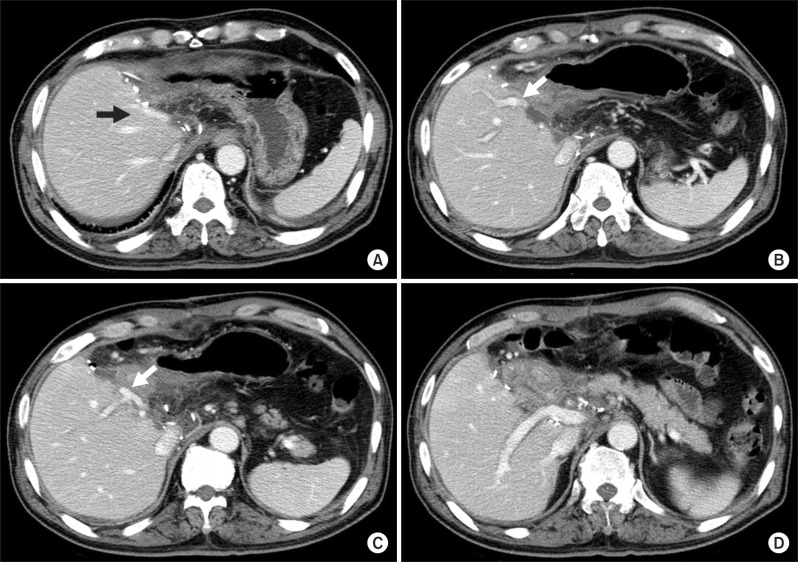 | Fig. 5Computed tomography images taken at 10 days after surgery. Two middle hepatic vein trunks are visible (A); The portal vein branch to the theoretical left medial section is visible (arrow, B); The portal vein branch to the right anterior section is visible and an arrow indicates the site of branch patch repair (C); The portal vein branch to the right posterior section is visible (D).
|
The caudate duct did not appear to be involved based on the preoperative imaging study findings, but the Spigelian lobe with the paracaval portion was excised to achieve maximal curability according to the general concept of surgical resection for perihilar cholangiocarcinoma. One 5-mm-sized bile duct opening was exposed following caudate lobe resection, but it was directly communicating with the right-sided duct; therefore, it was closed because bilio-enteric reconstruction was not necessary. Locoregional lymph nodes were dissected from the hepatic hilum to the celiac axis because some nodes were tumor-positive on frozen-section biopsy. Biliary reconstruction was performed through a single, large hepaticojejunostomy using a Roux-en-Y limb.
Pathological examination revealed that the final diagnosis was moderately differentiated adenocarcinoma involving the liver with vascular invasion of the left hepatic artery and left portal vein, concurrent perineural invasion without lymphovascular invasion, and lymph node metastasis in 5 out of 12 nodes (
Fig. 6). Thus, tumor staging was T3N1M0, being stage IIIB according the 7th edition of the American Joint Committee on Cancer (AJCC) staging system.
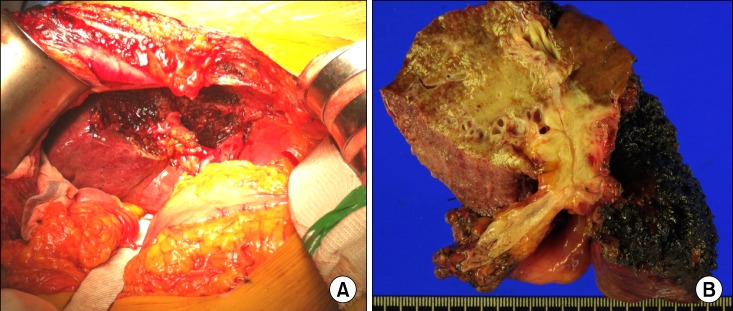 | Fig. 6Gross photographs of the operative field after resection (A) and the resected specimen (B).
|
The patient recovered uneventfully without any complication and was discharged 11 days after surgery. Currently, the patient is receiving concurrent chemoradiation therapy.
Go to :

DISCUSSION
According to our experience and the literature, the presence of LSGB is often associated with serious combined anomalies of the hepatic vascular and biliary systems. Such a constellation of combined hepatic anomalies can make liver surgery difficult; therefore, the operation should be prudently planned with accurate preoperative evaluation. Besides complete preoperative evaluation, the absence of anatomical variations contraindicating the pre-planned extent of hepatectomy should be confirmed intraoperatively.
In adult individuals with LSGB, portal anomaly must be the most significant part in the constellation of multiple anomalies.
123456 Nagai et al.
3 reviewed collectively the anatomical variations combined with LSGB in their own 3 cases of living donors and previously reported 15 cases. The patterns of intrahepatic portal venous branching were divided into the bifurcation type (5 cases), the trifurcation type (8 cases), and undetermined (5 cases) due to lack of detailed description. We also presented 3 cases of living donors with LSGB, the bifurcation type was found in two cases and the trifurcation type was found in one case [1]. The present case appeared to be of the trifurcation type although there was some dissimilarity.
The anatomy of the hepatic artery in individuals with LSGB has not been described in detail in the literature, but we presume that it runs along the course of the portal vein branches. Variation of the hepatic artery is often different from that of the portal vein. Bile duct anatomy in individuals with LSGB has also not been described in detail elsewhere. Based on our experience of 4,000 living donor hepatectomies, the rarer the type of the portal vein variation was, the more common the combined bile duct variation became. Variant portal vein anatomy such as type III variation is usually accompanied by the uncommon types of bile duct variation.
7
In patients with LSGB who were diagnosed with perihilar cholangiocarcinoma, the anatomy of the biliary system must be seriously distorted; therefore, it is important to imagine the premorbid variant biliary anatomy and then assess the extent of tumor invasion. If the present patient had usual liver anatomy, it might not have been possible to achieve macroscopic curative resection because the tumor might have invaded the portal confluence as well as the right hepatic artery. In fact, the anomalous anatomy in LSGB might be helpful for resecting the tumor because it might displace the location of the tumor towards the peripheral side of the left hepatic duct. Such a tumor location is rare in the usual patients with perihilar cholangiocarcinoma. Probably due to such unusual location of the tumor, the caudate duct was free of tumor invasion. From a retrospective viewpoint, concurrent caudate lobe resection did not appear to be necessary in the present patient. However, during operation, we intended to achieve maximal macroscopic curability; therefore, caudate lobe resection was carried out as it is routinely performed in other patients with perihilar cholangiocarcinoma.
In conclusion, we experienced a case of a patient with perihilar cholangiocarcinoma and concurrent anomalies of LSGB, and based on the preoperative and intraoperative assessment of the liver anatomy and tumor extent, a customized extended liver resection of the extended left lateral section and bile duct resection were accomplished safely without any postoperative complication. LSGB should be recognized as a constellation of multiple hepatic anomalies, and therefore, thorough investigations are necessary to enable the performance of safe hepatic and biliary resections.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download