This article has been
cited by other articles in ScienceCentral.
Abstract
Backgrounds/Aims
Pneumatosis intestinalis (PI) is a condition in which multiple gas-filled mural cysts develop in the gastrointestinal tract. Although its exact etiology remains obscure, PI is rarely observed in liver transplant (LT) recipients.
Methods
In 317 cases of adult living donor LT (LDLT) performed during 2011, PI developed in three patients during the 3 year follow-up.
Results
Of these three patients, the two who demonstrated PI at 6 weeks and 2 months after LT, respectively, were asymptomatic and showed no signs of secondary complications. Diagnosis was made incidentally using abdominal radiographs and computed tomography (CT) scans. PI was identified in the right ascending colon with concomitant pneumoperitoneum. These two patients received supportive care and maintained a regular diet. Follow-up CT scans demonstrated spontaneous resolution of PI with no complications. The third patient was admitted to the emergency room 30 months after LDLT. His symptoms included poor oral intake and intermittent abdominal pain with no passage of gas. Abdominal radiography and CT scans demonstrated PI in the entire small bowel, with small bowel dilatation, pneumoperitoneum, and pneumoretroperitoneum, but no peritonitis. Physical examination revealed abdominal distension but no tenderness or rebound tenderness. After 1 week of conservative treatment, including bowel rest and antibiotics therapy, PI and pneumoperitoneum resolved spontaneously without complications.
Conclusions
We suggest that adult LDLT recipients who develop asymptomatic or symptomatic PI with no signs of secondary complications can be successfully managed with conservative treatment.
Go to :

Keywords: Pneumatosis intestinalis, Living donor liver transplantation, Pneumoperitoneum
INTRODUCTION
Pneumatosis intestinalis (PI) is a condition in which multiple gas-filled mural cysts develop in the gastrointestinal tract,
1 and is characterized by accumulation of gas in the submucosa or subserosa of the colon or small bowel. In a retrospective review published by Koss in 1952,
2 15% of cases were classified as primary or idiopathic PI, 75% were considered secondary PI, and 10% had an unknown underlying disease. The majority of secondary PI cases are related to gastrointestinal disorders.
3 Although PI has been observed occasionally in recipients of liver transplantation (LT), the exact etiology remains obscure.
456 PI is diagnosed via computed tomography (CT) scan and simple abdomen radiographs and is managed surgically in most cases, despite high rates of mortality associated with surgery (33-44%).
7
Because the clinical presentation of PI after LT ranges widely from asymptomatic to fatal, we present our PI cases that occurred after LT with collective literature review.
Go to :

MATERIALS AND METHODS
During one year of 2011, we performed 403 LT operations, of which 317 were adult living donor LT (LDLT). PI developed in three patients (0.94%) who underwent LDLT and was successfully treated with conservative treatment. The clinical courses of these PI patients were retrospectively analyzed through the review of medical records. This study was approved by the Institutional Review Board of Asan Medical Center.
Go to :

RESULT
Case 1 presentation
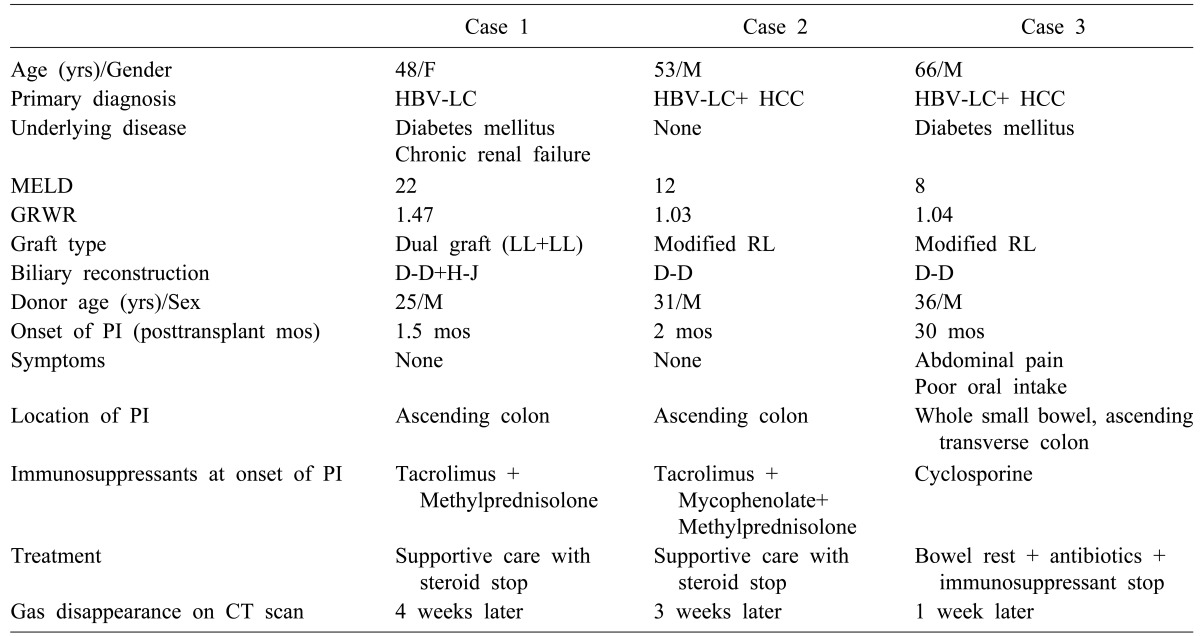
A 48-year-old woman with underlying diabetes mellitus and chronic renal failure had undergone dual-graft LDLT using two left lobes due to hepatitis B virus (HBV)- associated liver cirrhosis (LC). Biliary reconstruction was performed using duct-to-duct anastomosis in the right graft and hepaticojejunostomy in the left graft (
Table 1). Six weeks after LDLT, PI was diagnosed incidentally on abdomen radiographs and CT scans, which demonstrated PI in the right ascending colon with small pneumoperitoneum (
Fig. 1). The patient showed no symptoms associated with PI and no sign of secondary complications such as peritonitis, bowel ischemia, or perforation. She was maintained on a regular diet and was not administered antibiotics. Routine immunosuppressive agents, except for steroids, were continued. After 4 weeks, a follow-up CT scan demonstrated spontaneous resolution of PI with no complications.
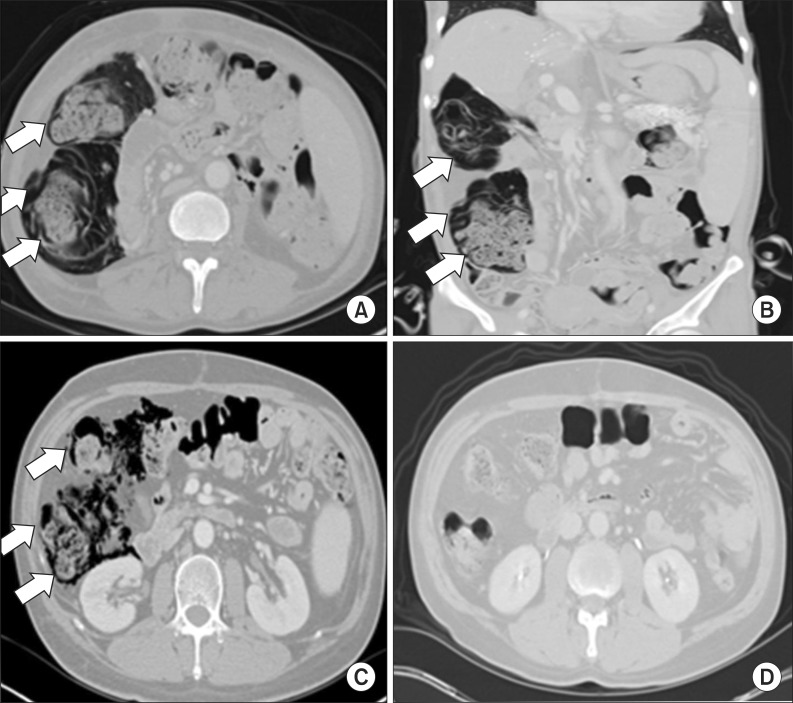 | Fig. 1Imaging of the case 1 and 2: Computed tomography (CT) scans of case 1 patient revealed pneumatosis intestinalis (PI) (white arrows) involving the right ascending colon on an axial image (A) and a coronal image (B). CT scans of case 2 patient revealed PI (white arrows) involving the right ascending colon (C), which was spontaneously resolved after 3 weeks (D).
|
Table 1
Clinical features and treatment for liver transplant recipients showing pneumatosis intestinalis (PI)

Case 2 presentation
A 53-year-old man with no underlying diseases underwent LDLT using a modified right lobe due to HBV-associated LC and hepatocellular carcinoma (HCC). The biliary reconstruction method was duct-to-duct anastomosis (
Table 1). After 2 months, diagnosis of PI was made incidentally on a routine CT scan, which demonstrated PI in the right ascending colon with small pneumoperitoneum (
Fig. 2). The patient had no symptoms associated with PI and no sign of secondary complications, and was well maintained on a regular diet without specific antibiotic therapy. Routine immunosuppressive agents, except for steroids, were maintained. A follow-up CT scan after 3 weeks revealed spontaneous resolution of PI with no complications.
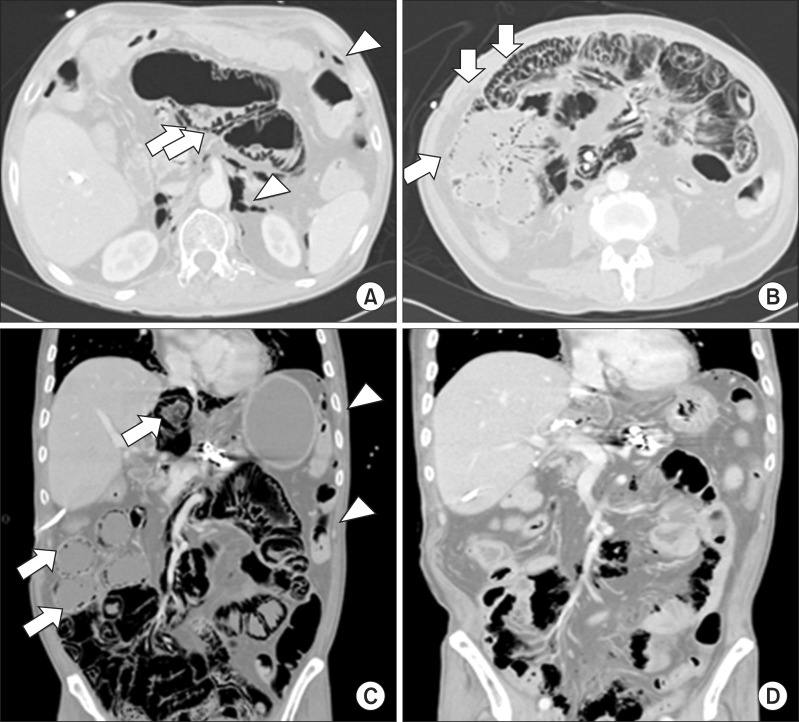 | Fig. 2Imaging of the case 3: Computed tomography (CT) scan demonstrated pneumatosis intestinalis (white arrows) involving the entire bowel with pneumoperitoneum (white arrowheads) and pneumoretroperitoneum (white arrowheads) (A-C). After 1 week of treatment, gas was almost completely resolved (D).
|
Case 3 presentation
A 66-year-old man with previous diagnosis of diabetes mellitus was admitted to the emergency room 30 months after LDLT due to HBV-associated LC and HCC. He had undergone a LDLT operation using a modified right lobe with a biliary reconstruction of Roux-en-Y hepaticojejunostomy (
Table 1). The patient had undergone explorative abdominal surgery for small bowel internal herniation 6 months before LT. At admission, he complained of poor oral intake and intermittent abdominal pain with no passage of gas. Abdomen radiographs and a CT scan demonstrated PI in the entire small bowel and right ascending and transverse colon, along with small bowel dilation, pneumoperitoneum, and pneumoretroperitoneum. The patient's vital signs were stable, and laboratory findings indicated mild leukocytosis (10,200/mm
3) and slightly elevated C-reactive protein (4.51 mg/dl), but no evidence of lactic acidosis or peritonitis. Physical examination revealed abdominal distension but no tenderness or rebound tenderness. After 1 week of conservative treatment that included bowel resting, antibiotics therapy, and withdrawal of immunosuppressive agents, PI and pneumoperitoneum spontaneously resolved without any complications. No other abnormalities occurred for 1 year following initial presentation.
Go to :

DISCUSSION
PI has been reported rarely in patients who have undergone LT.
4568 It is characterized by the following symptoms in decreasing order of frequency: diarrhea, abdominal pain, abdominal distension, bloody stool, constipation, weight loss, and tenesmus.
9 Several investigators have suggested that PI after solid organ transplantation is likely benign and precipitated by pre-transplantation chemotherapy and radiotherapy, immunosuppressive therapy (most notably steroid-based treatments), opportunistic enteric infections (particularly cytomegalovirus), and sympathetic reaction to inflamed allograft.
4689 Additionally, glucocorticoid therapy alone may significantly increase the risk of PI development because glucocorticoids may induce atrophy of gastrointestinal tract lymphoid aggregates,
10 which results in mucosal defects that allow dissection of intraluminal air/gas into the submucosal or subserosal regions.
11
PI is diagnosed via CT scan and simple abdomen radiography and is typically managed surgically, despite a high mortality rate (33-44%) associated with PI-related surgery.
7 CT scan is a more sensitive and specific test than simple abdomen radiography or ultrasonography.
12 Spontaneous pneumoperitoneum, which is frequently observed in PI, is presumably due to the rupture of subserosal cysts and usually does not worsen or turn into peritonitis.
4
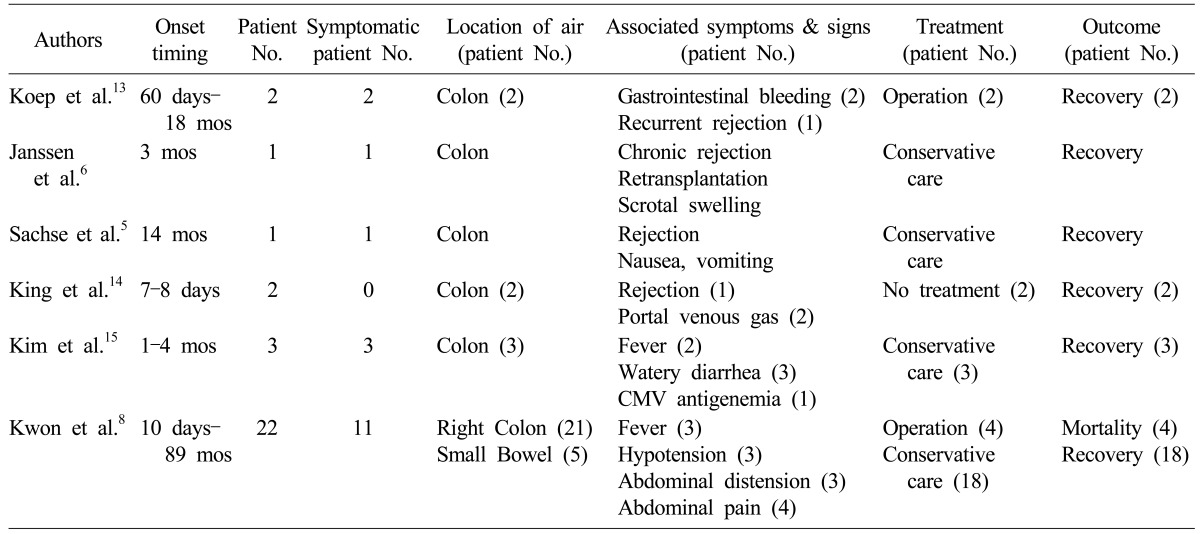
The literature review regarding PI after LT is summarized in
Table 2 and includes 31 PI cases.
568131415 Most patients recovered with supportive care, although one study of 22 cases reported four mortalities. These mortality cases showed fever, hypotension, and CT scan findings of infarcts at the spleen and liver, small bowel ileus, and hemorrhagic ascites.
8
Table 2
Collective review of liver transplant recipients showing pneumatosis intestinalis

In our study, the incidence of PI after adult LDLT was approximately 1%, and all three cases showed involvement of the right ascending colon. One patient had symptoms of abdominal pain and poor oral intake, whereas the other patients were asymptomatic. The former had received only low-dose cyclosporine as immunosuppressive therapy, while the others had received tacrolimus and steroids with or without mycophenolate mofetil.
The tendency of indolent PI to affect the right ascending colon has been demonstrated in previous studies of patients who received bone marrow transplantation.
16 Preservation of structural integrity in the right ascending colon may depend on immunocompetent lymphoid tissue, which may be impaired by immunosuppression and steroid therapy. This impairment may be a precondition to the development of PI in LT patients and may explain why all of our patients experienced PI in the right ascending colon.
817
Although our study is limited by the small sample size, the findings suggest that if patients develop PI after LDLT but exhibit no secondary complications, they can be successfully managed with conservative treatment that includes bowel rest, antibiotic therapy, and withdrawal of immunosuppressive agents.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download