Abstract
Backgrounds/Aims
Liver resection is a curative procedure performed worldwide for hepatocellular carcinoma (HCC). Deciding on the appropriate resection range for postoperative hepatic function preservation is an important surgical consideration. This study compares survival outcomes of HCC patients who underwent anatomical or non-anatomical resection, to determine which offers the best clinical survival benefit.
Methods
One hundred and thirty-one patients underwent liver resection with HCC, between January 2007 and February 2015, and were divided into two groups: those who underwent anatomical liver resection (n=88) and those who underwent non-anatomical liver resection (n=43). Kaplan-Meier survival analysis and Cox regressions were used to compare the disease-free survival (DFS) and overall survival (OS) rates between the groups.
Results
The mean follow-up periods were 27 and 40 months in the anatomical and non-anatomical groups, respectively (p=0.229). The 3- and 5-year DFS rates were 70% and 60% in the anatomical group and 62% and 48% in the non-anatomical group, respectively. The 3 and 5-year OS rates were 94% and 78% in the anatomical group, and 86% and 80% in the non-anatomical group, respectively. The anatomical group tended to show better outcomes, but the findings were not significant. However, a relative risk of OS between the anatomical and non-anatomical group was 0.234 (95% CI, 0.061-0.896; p=0.034), which is statistically significant.
Liver resection is a curative procedure performed worldwide for hepatocellular carcinoma (HCC).1 Anatomical resection is an especially effective curative treatment; however, massive anatomical liver resection can be difficult to execute in patients with existing liver conditions, such as hepatitis or hepatic cirrhosis. In the Korean population, one study reports that 74.6% of HCC patients were positive of Hepatitis B virus (HBV) and 9.3% were positive of hepatitis C virus (HCV).2 Further, it is predicted that one-third of cirrhotic liver patients will develop HCC.3 Because of this, to preserve liver function, there are cases in which limited liver resection is performed, irrespective of the anatomical structure of the liver.45
Because recurrence is common after HCC surgery, hepatic function at the time of recurrence is critical to determine the survival rate after recurrence. In particular, mortality after liver surgery can result from not only recurrence, but also decreased hepatic function and exacerbation of existing liver disease.6 Therefore, deciding on the appropriate resection range for preservation of postoperative hepatic function is important to make informed surgical treatment decision.
From oncological and anatomical perspectives, anatomical liver resection is theoretically superior to non-anatomical liver resection, however clinical studies have failed to show any differences in survival benefit between them.789
The present study examined 131 cases of liver resection performed by the authors with the aim of comparing disease-free survival (DFS) and overall survival (OS) between anatomical and non-anatomical resection, in order to determine which offers the best survival benefits.
The diagnosis of HCC was based on imaging modality, including enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and tumor markers. Considering cancer cell seeding during liver biopsy, preoperative liver biopsy was not suggested for all patients initially. Diagnosis of HCC mainly depended on typical findings: early-phase enhancement or late-phase contrast washout. Elevation of alpha-fetoprotein (AFP) and history of hepatic viral infection or heavy alcohol drinking were also considered. Before treatment, all patients underwent basal laboratory tests including bilirubin, albumin and prothrombin activity. To predict postoperative liver failure, an ICG test and fibroscan were performed.
Two hundred and twenty-seven patients diagnosed with HCC between January 2007 and February 2015 were evaluated. Thirty-four patients had undergone previous radiofrequency ablation (RFA), transcatheter arterial chemoembolization, or surgery after HCC diagnosis were excluded. Eighteen patients who had other cancers, such as cholangiocarcinoma or stomach cancer at the time of the operation were also excluded. An additional 44 patients who underwent concomitant RFA during liver resection were excluded. The remaining 131 patients were divided into two groups, those who underwent anatomical liver resection (n=88) and those who underwent non-anatomical liver resection (n=43).
Anatomical liver resection involved segment-oriented resection from the areas that included the tumor and where the hepatic portal veins and hepatic veins divided, following the terminology proposed by Strasberg.10 Non-anatomical liver resection involved resection of the lesion area regardless of anatomical segment or lobe. For a single HCC with well-preserved liver function and ≤15% of ICG R15, anatomical major liver resection was initially considered for curative treatment. For multiple tumors, anatomical major liver resection was considered when all tumors located in single lobe with good liver function. Non-anatomical liver resection was considered for a single tumor with suboptimal liver function or >15% of ICG R15 and for multiple tumors located in different lobes. In this study, a single segmental resection, such as S5 segmentectomy were not considered anatomical resection. Anatomical resection included right or left hemihepatectomy, right posterior or anterior sectionectomy, left lateral sectionectomy and central bisectionectomy.
Preoperative albumin, total bilirubin, prothrombin time international normalization ratio (PT [INR]), platelet count, tumor markers, and the presence of underlying liver diseases, such as hepatitis or hepatic cirrhosis, were compared between the groups. HCC staging was performed according to the American Joint Committee on Cancer/Union for International Cancer Control TNM staging guidelines.11 Histology was compared using Edmondson-Steiner grading.12
Clinical characteristics and perioperative factors between the anatomical and non-anatomical groups were analyzed using an independent sample t-test. DFS and OS were analyzed using the Kaplan-Meier method and the relative ratio was analyzed using the Cox regression model. Statistical analyses were conducted using SPSS software (version 19, SPSS Inc., Chicago, IL).
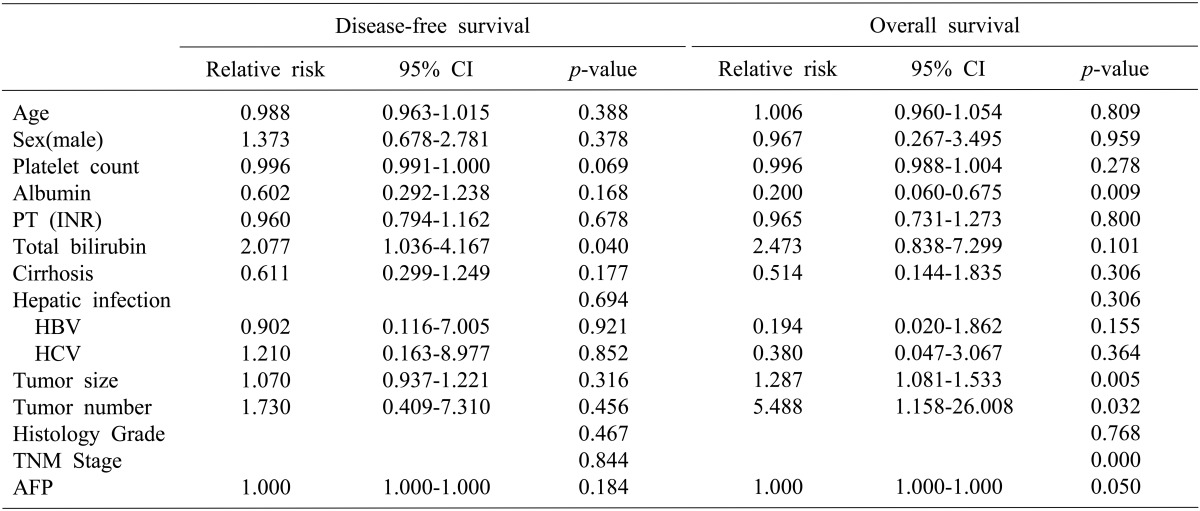
Patient characteristics are shown in Table 1. There were no significant differences in age, sex, total bilirubin, tumor number or PT-INR between the groups. In addition, platelet counts and serum albumin levels were statistically different; however, there was no difference in liver function of between the results of the two groups. Nevertheless, tumor size in the anatomical group was significantly larger than that in the non-anatomical group (p=0.004). Most patients had chronic hepatitis or cirrhosis as an underlying disease and most were hepatitis B virus positive. Histologic grades in all patients were mostly grade II or III. The majority of patients had TNM stage I or II disease. Alfa-fetoprotein levels were not evaluated in all patients, although there appeared to be no significant differences in patients whose levels were measured between the groups.
The mean follow-up periods were 27 and 40 months in the anatomical and non-anatomical groups, respectively (p=0.229). As shown in Fig. 1, the 3- and 5-year DFS rates were 70% and 60% in the anatomical group and 62% and 48% in the non-anatomical group, respectively. The 3 and 5-year OS rates were 94% and 78% in the anatomical group and 86% and 80% in the non-anatomical group, respectively. Although, the anatomical group tended to have better DFS and OS outcomes, the differences between the groups were not significant.
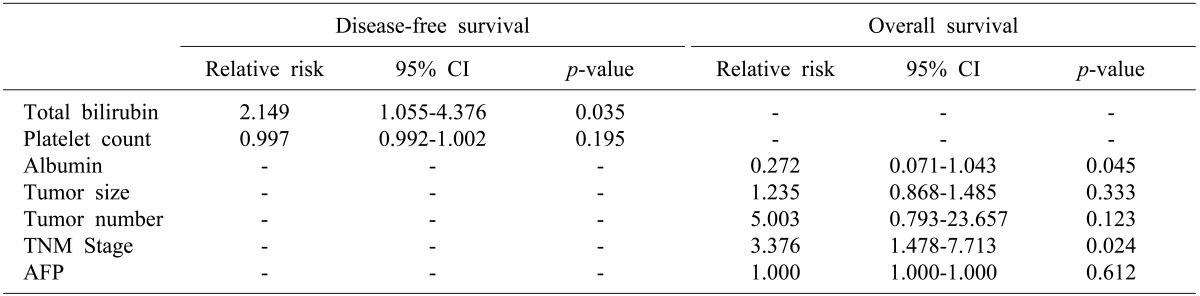
Univariate analysis of relative risk was performed on the perioperative factors analyzed (Table 2). For DFS, total bilirubin and platelet counts were significant predictors. However, a multivariate analysis showed that total bilirubin was a significant predictor of DFS (Table 3). In addition, a relative risk (RR) of DFS between the anatomical and non-anatomical groups was 0.804 (95% confidence interval [CI], 0.423-1.529; p=0.506), which is not statistically significant (Fig. 2). Serum albumin, tumor number, tumor size, stage and alfa-fetoprotein (AFP) were significant predictors of OS in univariate analysis. Furthermore, a multivariate analysis showed that serum albumin and stage were also significant predictors. RR of OS between the anatomical and non-anatomical groups was 0.234 (95% CI, 0.061-0.896; p=0.034), which is statistically significant (Fig. 2).
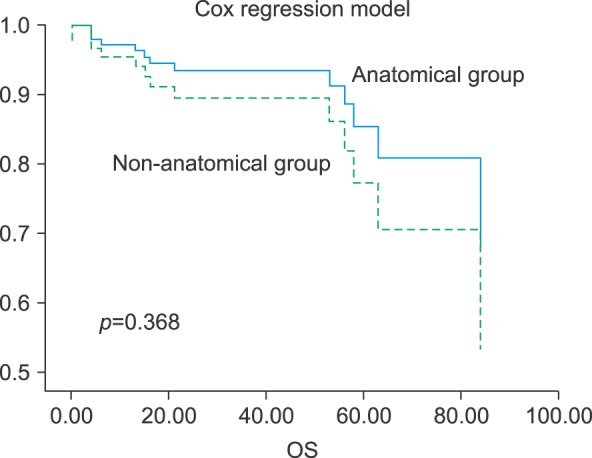
In subjects with a single tumor, anatomical resection groups showed better overall survival in the graph, however, there was no statistical difference of two groups (Fig. 3).
Liver resection is a curative procedure for HCC. In particular, eradication of intrahepatic metastasis occurring via vascular invasion is one of the most important considerations. Therefore, the non-anatomic approach is disadvantageous when considered from the standpoint of intrahepatic metastasis eradication.13 Retrospective studies report that a three year survival rate with anatomical resection is better than with limited non-anatomical resection.14
However, only a few patients with HCC undergo liver resection because of poor liver function such as cirrhosis, chronic liver disease, and the difficulty in predicting postoperative liver failure.45 Although anatomical resection for HCC treatment is being recommended more often,1315 recently conducted studies fail to demonstrate any distinct differences in DFS or OS between anatomical resection and non-anatomical resection.789 Furthermore, some surgeons prefer to leave a greater portion of parenchyma of functional unit, such as in non-anatomic resection because most tumors arise in cirrhotic livers.16 In other studies, non-anatomical resection is equal to anatomical resection and in some cases, non-anatomical hepatic resection is recommended over anatomical resection.1718
Some studies indicate that very restrictive patient selection is required to perform anatomical resection. Although it is especially recommended for Child-Pugh class A patients19 or non-cirrhotic liver20 patient groups, it is reported that there is no difference in the improvement of survival or recurrence rates compared with those after non-anatomical resection in these patients.15
From an operative results perspective, although securing the resection margin is more difficult in non-anatomical resection compared with anatomical resection,7 the resection volume of the normal liver parenchyma may be bigger in some anatomical resection cases. This suggests that a smaller liver volume can be removed to achieve the same margin status in cases of non-anatomical resection, which may be more favorable for postoperative liver function.21 Takano et al.7 report that partial resection is associated with a reduced frequency of operative complications, more so than anatomical resection, although there were no differences in morbidity rates between them. In addition, they demonstrated that non-anatomical resection offered more benefits in terms of hepatic parenchyma preservation, which allows for additional surgery or other treatments in cases of recurrence. From a liver function perspective, Shirabe et al.22 report that patients with better postoperative liver function lived >10 years longer compared to those with poor liver function. They emphasized the importance of preserving hepatic function at the time of operation because survival was affected by underlying liver conditions.
Various studies have published the benefit of anatomical resection or non-anatomical resection for the patients with preserved liver function. Nagasue et al.23 analyzed the outcome of anatomical liver resection and partial liver resection for patients with HCCs with preserved liver function (Child-Pugh A). Moreover, there were no significant differences in DFS and survival between the operation methods. On the other hand, Hasegawa et al.13 analyzed the outcomes of anatomical and non-anatomical resection for HCC in Child-Pugh A and B groups. The five-year survival and DFS rates were better in the anatomical resection (Survival, 66% vs. 35%; DFS, 34% vs. 16%).
In the present study, the anatomical resection group showed better DFS and OS outcomes than the non-anatomical resection group, although the results of DFS and OS were not statistically significant. Further, the anatomical resection group showed results that are more beneficial in relative risk of OS. The reason there is no statistical difference may be due to the difference between the follow-up periods of the two groups. However, liver function of our patients was well preserved after the patients underwent liver resection. Furthermore, there were no major differences in clinical characteristics and perioperative factors between the two groups. Therefore, if there was no difference in the follow-up period, we can expect that anatomical resection can provide better survival outcomes when there are no major differences in underlying liver conditions and well preserved liver function.
The tumor number and TNM stage in the relative risk of overall survival showed significant results in the univariate study. However, tumor number in the multivariate analysis was not statistically significant. If the number of tumors is more diverse, it is expected to have a significantly better result.
As described above, our study had limitations. First, there was a large discrepancy in the mean follow-up duration between the groups. Second, the sample size was small. Nevertheless, we believe that significant results can be derived in longer-term studies with a greater number of patients.
In conclusion, although statistical significance was not detected in survival curves, anatomical resection showed better results. In this respect, anatomical resection should be considered before non-anatomical resection for HCC patients with well-preserved liver function.
References
1. Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995; 221:291–298. PMID: 7717783.

2. Kwak HW, Park JW, Nam BH, Yu A, Woo SM, Kim TH, et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2014; 29:820–829. PMID: 24325272.

3. Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006; 43:1303–1310. PMID: 16729298.

4. Kanematsu T, Takenaka K, Matsumata T, Furuta T, Sugimachi K, Inokuchi K. Limited hepatic resection effective for selected cirrhotic patients with primary liver cancer. Ann Surg. 1984; 199:51–56. PMID: 6318677.

5. Bismuth H, Houssin D, Ornowski J, Meriggi F. Liver resections in cirrhotic patients: a Western experience. World J Surg. 1986; 10:311–317. PMID: 3010585.

6. Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993; 18:1121–1126. PMID: 8225217.

7. Takano S, Oishi H, Kono S, Kawakami S, Nakamura M, Kubota N, et al. Retrospective analysis of type of hepatic resection for hepatocellular carcinoma. Br J Surg. 2000; 87:65–70. PMID: 10606913.

8. Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003; 237:536–543. PMID: 12677151.
9. Kondo K, Chijiiwa K, Makino I, Kai M, Maehara N, Ohuchida J, et al. Risk factors for early death after liver resection in patients with solitary hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2005; 12:399–404. PMID: 16258809.

10. Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden Jo, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. Terminology Committee of the International Hepato-Pancreato-Biliary Association. HPB. 2000; 2:333–339.
11. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010. p. 175.
12. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954; 7:462–503. PMID: 13160935.
13. Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005; 242:252–259. PMID: 16041216.

14. Nagao T, Inoue S, Goto S, Mizuta T, Omori Y, Kawano N, et al. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987; 205:33–40. PMID: 3026259.
15. Fuster J, García-Valdecasas JC, Grande L, Tabet J, Bruix J, Anglada T, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg. 1996; 223:297–302. PMID: 8604911.
16. Cucchetti A, Cescon M, Ercolani G, Bigonzi E, Torzilli G, Pinna AD. A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol. 2012; 19:3697–3705. PMID: 22722807.

17. Marubashi S, Gotoh K, Akita H, Takahashi H, Ito Y, Yano M, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. 2015; 102:776–784. PMID: 25847111.

18. Tomimaru Y, Eguchi H, Marubashi S, Wada H, Kobayashi S, Tanemura M, et al. Equivalent outcomes after anatomical and non-anatomical resection of small hepatocellular carcinoma in patients with preserved liverfunction. Dig Dis Sci. 2012; 57:1942–1948. PMID: 22407377.
19. Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002; 131:311–317. PMID: 11894036.

20. Kosuge T, Makuuchi M, Takayama T, Yamamoto J, Shimada K, Yamasaki S. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology. 1993; 40:328–332. PMID: 8406301.
21. Tanaka K, Shimada H, Matsumoto C, Matsuo K, Nagano Y, Endo I, et al. Anatomic versus limited nonanatomic resection for solitary hepatocellular carcinoma. Surgery. 2008; 143:607–615. PMID: 18436008.

22. Shirabe K, Shimada M, Kajiyama K, Gion T, Ikeda Y, Hasegawa H, et al. Clinicopathologic features of patients with hepatocellular carcinoma surviving >10 years after hepatic resection. Cancer. 1998; 83:2312–2316. PMID: 9840530.
23. Nagasue N, Yamanoi A, el-Assal ON, Ohmori H, Tachibana M, Kimoto T, et al. Major compared with limited hepatic resection for hepatocellular carcinoma without underlying cirrhosis: a retrospective analysis. Eur J Surg. 1999; 165:638–646. PMID: 10452257.
Fig. 1
Disease-free and overall survival of the anatomical and non-anatomical groups by the Kaplan-Meier Method.
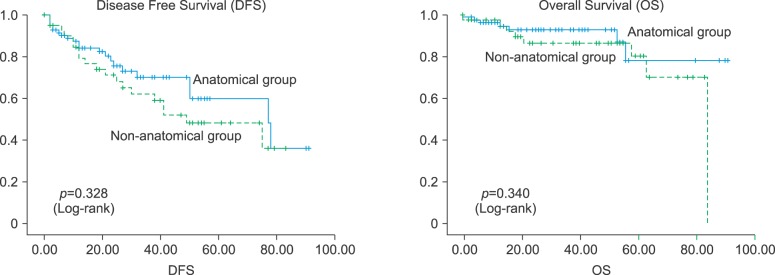
Fig. 2
Disease-free and overall survival of the anatomical and non-anatomical groups by the Cox regression model.
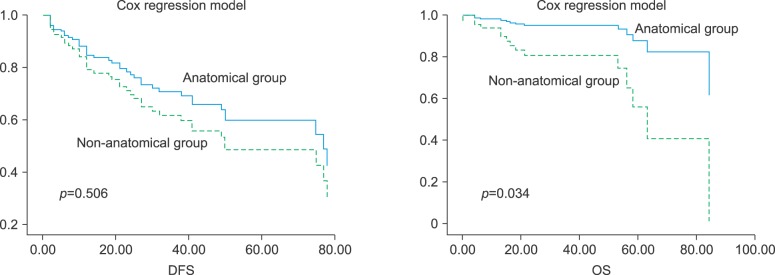
Fig. 3
Overall survival of the anatomical and non-anatomical group by the Cox regression model in patients with single tumor.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download