Abstract
Backgrounds/Aims
According to 7th AJCC TNM staging system, gallbladder carcinoma (GBC) with lymph node (LN) metastasis is classified as N1 or N2; thus making the stage IIIB (N1) or IVB (N2). Stage IIIB consists of N1 status with wide coverage of T1-3, but T3N1 group often showed poorer outcomes than T1-2N1 groups. This study intended to assess post-resection prognosis of T3N1 versus other stage III subgroups.
Methods
We selected 103 patients from our institutional database of GBC who underwent R0 resection between July 1996 and June 2009 and whose GBC was confined to stage T3N0, T1-3N1 or T1-3N2. These patients were stratified into five groups, namely, T3N0 (n=26), T1N1 (n=13), T2N1 (n=35), T3N1 (n=20) and T1-3N2 (n=9), and were followed for ≥5 years or until death.
Results
Surgical procedures were minor liver resection (n=53), minor liver resection with bile duct resection (n=23), major liver resection (n=12), major liver resection with bile duct resection (n=5), and hepatopancreatoduodenectomy (n=12). Mean follow-up period was 57.2±68.5 months. Overall 5-year survival rate based on all-cause death and cancer-associated death, respectively, was 57.7% and 60.6% in T3N0, 15.4% and 15.4% in T1N1 (n=13), 28.6% and 28.6% in T2N1 (n=35), 5.0% and 5.7% in T3N1 (n=20), and 22.2% and 22.2% in T1-3N2. The survival outcome of T3N1 group was the poorest among the four stage III groups and was comparable to that of stage IVB (p=0.53).
Conclusions
The prognosis of T3N1 GBC is unusually poor even after R0 resection, thus we suggest extensive LN dissection may be necessary in patients with T3 tumors for accurate prognostic evaluation and radical removal of potential nodal micrometastasis. Further validation of this result is necessary in large patient populations from multiple centers.
Go to : 
Gallbladder carcinoma (GBC) is a relatively rare malignancy and its prognosis depends primarily on the extent of tumor and the possibility of curative surgical resection. Due to the anatomical proximity of the gallbladder to the liver and other organs and to the unique patterns of tumor cell spread, resection for advanced GBC requires aggressive surgical approaches.1234 For stage T2 or more advanced tumors, radical resection includes partial hepatectomy and wide lymph node (LN) dissection, with or without bile duct resection.
To estimate the survival outcomes after curative resection of GBC, several staging systems were proposed. The American Joint Committee on Cancer (AJCC) staging system, which differentiates GBC based on the depth of invasion (T stage), LN metastasis (N stage) and distant metastasis (M stage), has been widely adopted. According to the 7th edition of the AJCC TNM staging system for GBC, LNs are divided into hilar LNs (N1) and other regional LNs (N2) and thus the presence of LN metastasis divides the tumor stage into IIIB (N1) and IVB (N2).5 In GBC of stage IIIB, the LN status becomes the most important prognostic factor with wide coverage of stage T1-3. However, in practice, we experience that a considerable proportion of GBC patients with N1 metastasis show unusually poor outcomes even after curative resection, especially patients with stage T3N1.
This study assesses the prognosis of T3N1 GBC versus that of other GBC stage III groups, and focuses on the prognostic impact of depth of invasion coupled with regional LN metastasis.
Go to : 
From our institutional database of GBC, which contains the records of more than 700 patients who underwent surgical resection, we selected 103 patients who underwent R0 resection (macroscopically curative resection with tumor-negative resection margins). between July 1996 and June 2009 and whose GBC was confined to stage III (T3N0 and T1-3N1) according to the 6th AJCC TNM staging system.5 Considering that the 7th AJCC staging system has been effective since January 2010,6 the extent of LN metastasis was reclassified retrospectively as N1 or N2 after thorough review of the surgical records, pathological reports and imaging studies. Therefore, the patients were reclassified as T3N0, T1-3N1 or T1-3N2 according to the 7th AJCC TNM staging system. Perioperative mortality within 1 month and reoperation cases following laparoscopic or open cholecystectomy were excluded from this study. Medical records were reviewed retrospectively after approval by the Institutional Review Board of our institution.
The study patients were followed up until July 2014, resulting in a follow-up period of ≥5 years or until patient death. No patient was censored during survival analysis because the survival status of all of the patients could be identified through the assistance of National Health Insurance Service.
The 103 patients were classified according to the 7th AJCC TNM staging system, as follows: 26 patients were T3N0M0 (stage IIIA), 68 were T1-3N1M0 (stage IIIB: T1N1M0 n=13, T2N1M0 n=35, and T3N1M0 n=20), and 9 were T1-3N2M0 (stage IVB: T1N2M0 n=2, T2N2M0 n=3, and T3N2M0 n=4). For the purpose of this study, they were stratified into five groups as follows: T3N0 (n=26), T1N1 (n=13), T2N1 (n=35), T3N1 (n=20) and T1-3N2 (n=9).
For suspected T2 GBC lesions, the usual extent of the surgical procedure (extended radical cholecystectomy) included en bloc wedge resection of the gallbladder and its bed, regional LN (N1±N2 group) dissection, and optional extrahepatic bile duct resection.78 Surgery for more advanced GBC required wider resection of the liver, extrahepatic bile duct resection and extensive LN (N1+N2 group±para-aortic node) dissection. If necessary, hepatopancreatoduodenectomy was performed to remove pancreatic invasion and peripancreatic LNs. Para-aortic LN dissection was not performed routinely.
The primary endpoint of this study was overall patient survival based on cancer-related death. Numeric variables were presented as means with standard deviations or as medians with ranges. Patient deaths were divided into all-cause death and GBC recurrence-associated death. GBC-related patient survival was obtained after censoring cancer-unrelated death. Survival and tumor recurrence rates were determined by the Kaplan-Meier method and compared by the log-rank test. p<0.05 was considered statistically significant. Statistical analyses were performed using SPSS (version 20, IBM, USA) and Statistica (version 6.0, StatSoft, OK, USA).
Go to : 
The mean age was 61.6±10.0 years (range, 35-84) and female gender was predominant (n=67, 65.1%). According to the 7th AJCC TNM staging system, the T stages of the primary tumors were pT1 in 14 patients, pT2 in 28 patients, and pT3 in 51 patients. LN stages were pN0 in 26 patients, pN1 in 68 patients, and N2 in 9 patients.
The clinicopathological profiles of the five groups are presented in Table 1. The surgical procedures were minor liver resection (n=51), minor liver resection with bile duct resection (n=23), major liver resection (n=12), major liver resection with bile duct resection (n=5), and hepatopancreatoduodenectomy (n=12).
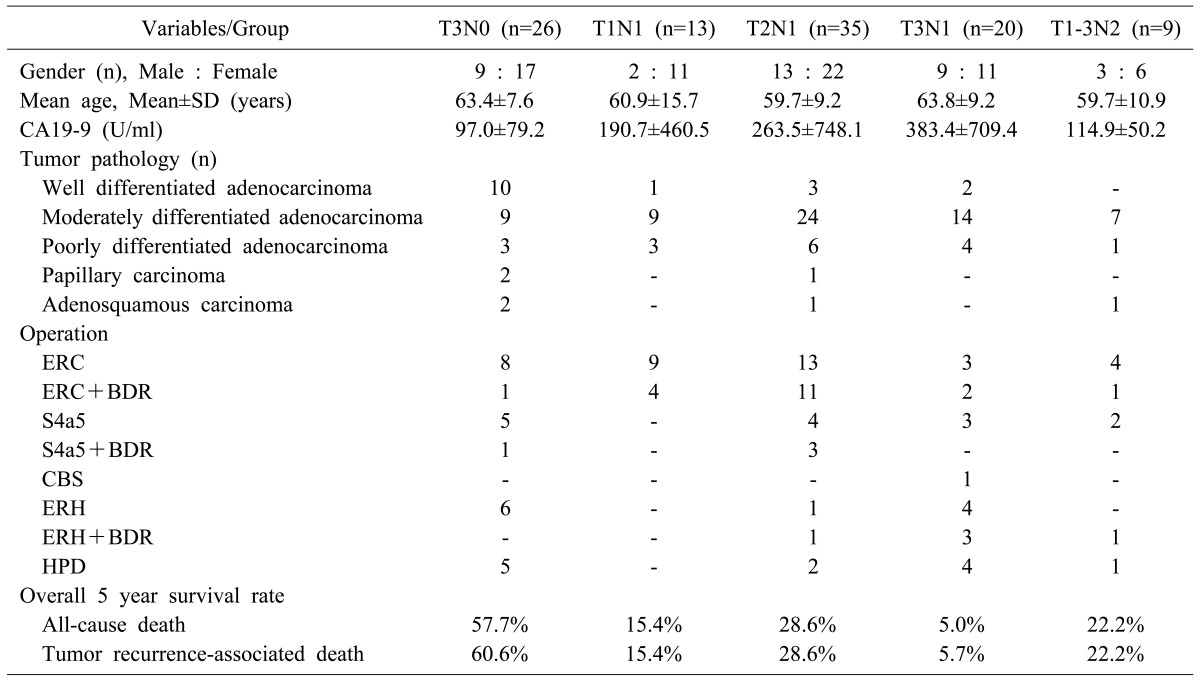
The mean and median follow-up periods were 57.2±68.5 months and 21 months, respectively. The overall survival curves of the five groups based on all-cause death are depicted in Fig. 1. At the end of follow-up, 23 patients were alive: 75 died from tumor recurrence and five died of other causes (other gastrointestinal malignancy in two patients, cerebrovascular accident in two patients and pneumonia in one patient). The overall 5-year survival rate based on all-cause death was 57.7% in T3N0 (n=26), 15.4% in T1N1 (n=13), 28.6% in T2N1 (n=35), 5.0% in T3N1 (n=20), and 22.2% in T1-3N2 (n=9: T1N2 in 1, T2N2 in 3 and T3N2 in 5).
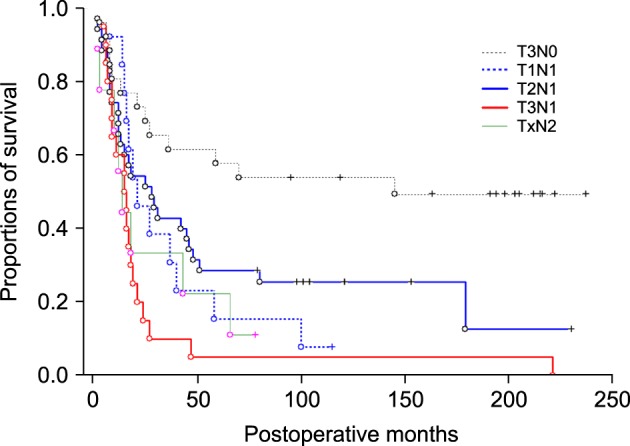
The overall survival curves based on GBC-related death are depicted in Fig. 2. The 5-year survival rates based on GBC-related death were 60.6% in T3N0, 15.4% in T1N1, 28.6% in T2N1, 5.7% in T3N1, and 22.2% in T1-3N2 (Table 1). Among the four groups in stage III, the survival outcome of the T3N1 group appeared the worst. The greatest survival difference was observed between the T3N0 and T3N1 groups (p<0.000). A noticeable survival difference was also observed between the T2N1 and T3N1 groups (p=0.031) and between the T1N1 and T3N1 groups (p=0.079). By contrast, there was no significant survival difference between the T1-3N2 (stage IVB) group and T3N1 group (stage IIIB) (p=0.534).
Go to : 
Nodal tumor cell spread is the most common form of progression of GBC, and LN status is an important predictor of survival after resection.9101112 In the 7th AJCC TNM staging system, LN classification was changed to distinguish hilar nodes (N1: LNs adjacent to the cystic duct, bile duct, hepatic artery and portal vein) from other regional nodes (N2: celiac, periduodenal and peripancreatic LNs and those along the superior mesenteric artery).6 This change might reflect the uniquely poor survival outcomes of patients with regional LN metastasis beyond the mid portion of the hepatoduodenal ligament.
In contrast to the 7th AJCC staging system, the Japanese Society of Biliary Surgery (JSBS) classification divided the LN status into three categories (N1: metastasis in cystic duct and/or pericholedochal LN; N2: metastasis in hepatoduodenal ligament except N1, posterosuperior pancreatic head, and/or along the common hepatic artery; N3: metastasis in peripancreatic [except posterosuperior pancreatic head], celiac, splenic, superior mesenteric, and/or para-aortic LNs).13 In a comparative study between the AJCC and JSBS systems, the T3N1 subgroup showed significantly worse survival outcome than the T2N1 subgroup regardless of staging systems.14
The present study revealed the prognostic impact of regional LN metastasis in patients who underwent R0 resection for advanced GBC. When there is no overt LN metastasis, a relatively favorable survival outcome would be anticipated after resection of locally advanced T3 stage tumor. By contrast, if the regional LNs was involved (stage T3N1), the survival outcome was far worsened and even similar to that of stage IVB patients. The typical recurrence pattern in patients with T3N1 GBCs was regional LN metastasis with or without intrahepatic metastasis. At this time, we presume that occult metastasis or micrometastasis may have been present in remote regional LNs at the time of surgery.151617 Sasaki et al.15 investigated the incidence of LN micrometastasis, which was detected immunohistochemically in 23 (34.3%) of 67 patients and in 37 (2.5%) of the 1,476 nodes examined. Survival outcome was worse in the 23 patients with micrometastasis than in the 44 patients without micrometastasis (5 year survival rates: 17.4% vs. 52.7%), and 28 patients without any LN involvement showed the best prognosis, whereas survival for the 11 patients with both types of metastasis was the worst. Therefore they suggested that LN micrometastasis has a significant survival impact in patients with GBC of N0 or N1 status who underwent macroscopically curative resection. Tanabe et al.16 reported that metastases were detected in 163 out of 1,094 LNs (14.9%) from 41 patients and that micrometastasis was found in 25 of the remaining LNs (2.3%). Micrometastasis and microscopic venous invasion were significant prognostic factors. These studies suggested that nodal micrometastasis is an independent risk factor in GBC after curative resection,151617 but that is occasionally not reflected on tumor staging because some LNs show only micrometastasis but not overt metastasis. It is recommended to perform extensive LN sectioning with immunohistochemical staining for accurate prognostic evaluation of patients with advanced GBC.15
In contrast to LN classification based on location, the number of positive LNs was reported to be an independent risk factor following resection of GBC.18 In a series of 116 GBC patients who underwent curative resection with dissection of 2,406 LNs, the 5-year survival rates were 81% for patients without nodal metastasis, 62% for patients with a single positive node, 43% for patients with 2-3 positive nodes, and 15% for patients with ≥4 positive nodes. No nodal disease or a single positive node indicates a favorable outcome after resection, whereas radical LN dissection is effective for selected patients with multiple positive nodes, provided that a R0 resection is feasible.
With the increasing safety of hepatic and pancreatic surgery, various radical procedures have been advocated to improve the curative outcome of advanced GBC. Aggressive resection may improve long-term survival, even in patients with far advanced GBC.18 Radical resection should not be abandoned for patients with para-aortic LN metastasis in biliary adenocarcinoma.19 However, unlike bile duct cancers, the survival outcome of GBC patients with N2 nodal metastasis has been usually dismal despite aggressive surgery, thus M1 nodal metastasis may not be indicated for aggressive resection.20 By contrast, adjuvant chemoradiotherapy improves overall survival in patients with advanced GBC having undergone resection.212223
The survival of the T1-2N1 subgroup is relatively favorable after aggressive resection, probably because extensive LN dissection may provide the curative removal of overt LN metastasis and potential micrometastasis. However, with regards to the poor survival of the T3N1 subgroup, we presume that the extent of LN dissection routinely performed may not be sufficient to remove all of the tumor cell spreads in the regional LNs.151617 Therefore, we suggest that more extensive LN dissection is necessary for T3 GBC tumors than for T1-2 tumors. Extensive resection, including resection of the duodenum and pancreatic head, is effective in selected patients with up to N2 lymph node metastasis as long as complete removal of the cancer can be achieved.24 In our study, hepatopancreatoduodenectomy was performed to obtain tumor-free resection margins or to remove peripancreatic nodes in 12 patients, among whom 10 had T3 tumors.
The present study has several limitations. First, this is a retrospective single-center study and thus the results may not be generalizable. In addition, some cases of N2 metastasis might have been underestimated and classified as N1 metastasis because LN classification was retrospectively redefined according to the 7th AJCC staging system. By contrast, a uniquely strong point of this study is that the survival status of all of the patients was nearly completely traced through the assistance of National Health Insurance Service. By contrast, this approach focused on survival status did not provide complete follow-up information regarding tumor recurrence and thus lack of tumor recurrence analysis becomes the weak point of this study.
In conclusion, the results of our study demonstrate that the prognosis of patients with T3N1 GBC is unusually poor and similar to that of N2 lesions. We suggest extensive LN dissection is necessary for GBC patients with T3 tumor for accurate prognostic evaluation and radical removal of potential nodal micrometastasis. Further validation of our result is necessary in large patient populations from multiple centers.
Go to : 
References
1. Wakai T, Shirai Y, Sakata J, Nagahashi M, Ajioka Y, Hatakeyama K. Mode of hepatic spread from gallbladder carcinoma: an immunohistochemical analysis of 42 hepatectomized specimens. Am J Surg Pathol. 2010; 34:65–74. PMID: 19956061.

2. Chikamoto A, Tsuji T, Nakahara O, Sakamoto Y, Ikuta Y, Tanaka H, et al. Cancer cells spread through lymph vessels in the submucosal layer of the common bile duct in gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2009; 16:557–561. PMID: 19373429.

3. Nakata T, Kobayashi A, Miwa S, Soeda J, Miyagawa S. Impact of tumor spread to the cystic duct on the prognosis of patients with gallbladder carcinoma. World J Surg. 2007; 31:155–161. PMID: 17180477.

4. Yang XW, Yang J, Li L, Man XB, Zhang BH, Shen F, et al. Analysis of the relationships between clinicopathologic factors and survival in gallbladder cancer following surgical resection with curative intent. PLoS One. 2012; 7:e51513. PMID: 23300551.

5. Oh TG, Chung MJ, Bang S, Park SW, Chung JB, Song SY, et al. Comparison of the sixth and seventh editions of the AJCC TNM classification for gallbladder cancer. J Gastrointest Surg. 2013; 17:925–930. PMID: 23299221.

6. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A 3rd. AJCC cancer staging manual. 7th ed. New York: Springer;2009. .
7. Araida T, Higuchi R, Hamano M, Kodera Y, Takeshita N, Ota T, et al. Should the extrahepatic bile duct be resected or preserved in R0 radical surgery for advanced gallbladder carcinoma? Results of a Japanese Society of Biliary Surgery Survey: a multicenter study. Surg Today. 2009; 39:770–779. PMID: 19779773.

8. Chikamoto A, Tsuji T, Nakahara O, Sakamoto Y, Ikuta Y, Tanaka H, et al. Cancer cells spread through lymph vessels in the submucosal layer of the common bile duct in gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2009; 16:557–561. PMID: 19373429.

9. Tsukada K, Kurosaki I, Uchida K, Shirai Y, Oohashi Y, Yokoyama N, et al. Lymph node spread from carcinoma of the gallbladder. Cancer. 1997; 80:661–667. PMID: 9264348.

10. Chijiiwa K, Yamaguchi K, Tanaka M. Clinicopathologic differences between long-term and short-term postoperative survivors with advanced gallbladder carcinoma. World J Surg. 1997; 21:98–102. PMID: 8943185.

11. Tsukada K, Hatakeyama K, Kurosaki I, Uchida K, Shirai Y, Muto T, et al. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery. 1996; 120:816–821. PMID: 8909516.

12. Todoroki T, Kawamoto T, Takahashi H, Takada Y, Koike N, Otsuka M, et al. Treatment of gallbladder cancer by radical resection. Br J Surg. 1999; 86:622–627. PMID: 10361182.

13. Japanese Society of Biliary Surgery. Classification of biliary tract carcinoma. 2nd ed. Tokyo: Kanehara;2004.
14. Kishi Y, Shimada K, Hata S, Oguro S, Sakamoto Y, Nara S, et al. Definition of T3/4 and regional lymph nodes in gallbladder cancer: which is more valid, the UICC or the Japanese staging system? Ann Surg Oncol. 2012; 19:3567–3573. PMID: 22890597.

15. Sasaki E, Nagino M, Ebata T, Oda K, Arai T, Nishio H, et al. Immunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with gallbladder carcinoma. Ann Surg. 2006; 244:99–105. PMID: 16794394.

16. Tanabe M, Endo I, Masunari H, Sugita M, Morioka D, Ishikawa T, et al. Is lymph-node micrometastasis in gallbladder cancer a significant prognostic factor. Hepatogastroenterology. 2012; 59:31–35. PMID: 22251520.
17. Nagakura S, Shirai Y, Yokoyama N, Hatakeyama K. Clinical significance of lymph node micrometastasis in gallbladder carcinoma. Surgery. 2001; 129:704–713. PMID: 11391369.

18. Kai M, Chijiiwa K, Ohuchida J, Nagano M, Hiyoshi M, Kondo K. A curative resection improves the postoperative survival rate even in patients with advanced gallbladder carcinoma. J Gastrointest Surg. 2007; 11:1025–1032. PMID: 17508256.

19. Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Is para-aortic lymph node metastasis a contraindication for radical resection in biliary carcinoma? World J Surg. 2011; 35:1085–1093. PMID: 21400012.

20. Nishio H, Nagino M, Ebata T, Yokoyama Y, Igami T, Nimura Y. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg. 2007; 14:351–357. PMID: 17653632.

21. Gold DG, Miller RC, Haddock MG, Gunderson LL, Quevedo F, Donohue JH, et al. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys. 2009; 75:150–155. PMID: 19297105.

22. Jeong Y, Park JH, Lee YJ, Park KM, Hwang S, Chang HM, et al. Postoperative radiotherapy for gallbladder cancer. Anticancer Res. 2014; 34:5621–5629. PMID: 25275065.
23. Murakami Y, Uemura K, Hayasidani Y, Sudo T, Hashimoto Y, Ohge H, et al. Indication for postoperative adjuvant therapy in biliary carcinoma based on analysis of recurrence and survival after surgical resection. Dig Dis Sci. 2009; 54:1360–1364. PMID: 18975086.

24. Sasaki R, Itabashi H, Fujita T, Takeda Y, Hoshikawa K, Takahashi M, et al. Significance of extensive surgery including resection of the pancreas head for the treatment of gallbladder cancer--from the perspective of mode of lymph node involvement and surgical outcome. World J Surg. 2006; 30:36–42. PMID: 16369715.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download