Abstract
Backgrounds/Aims
The aim of this study was to compare operative versus non-operative management of patients with liver injury and to ascertain the differences of the clinical features.
Methods
From April 2000 to July 2012, 191 patients were admitted to Seoul St. Mary's Hospital and St. Vincent's Hospital for liver injuries. Of these, 148 patients were included in this study. All patients were diagnosed using computed tomography (CT). The liver injury was graded in accordance with the American Association for the Surgery of Trauma liver injury scoring scale. Patients were divided into two groups: those who underwent surgery and those treated with non-operative management (NOM). There was a comparison between these two groups concerning the clinical characteristics, grade of liver injury, hemodynamic stability, laboratory findings, and mortality.
Results
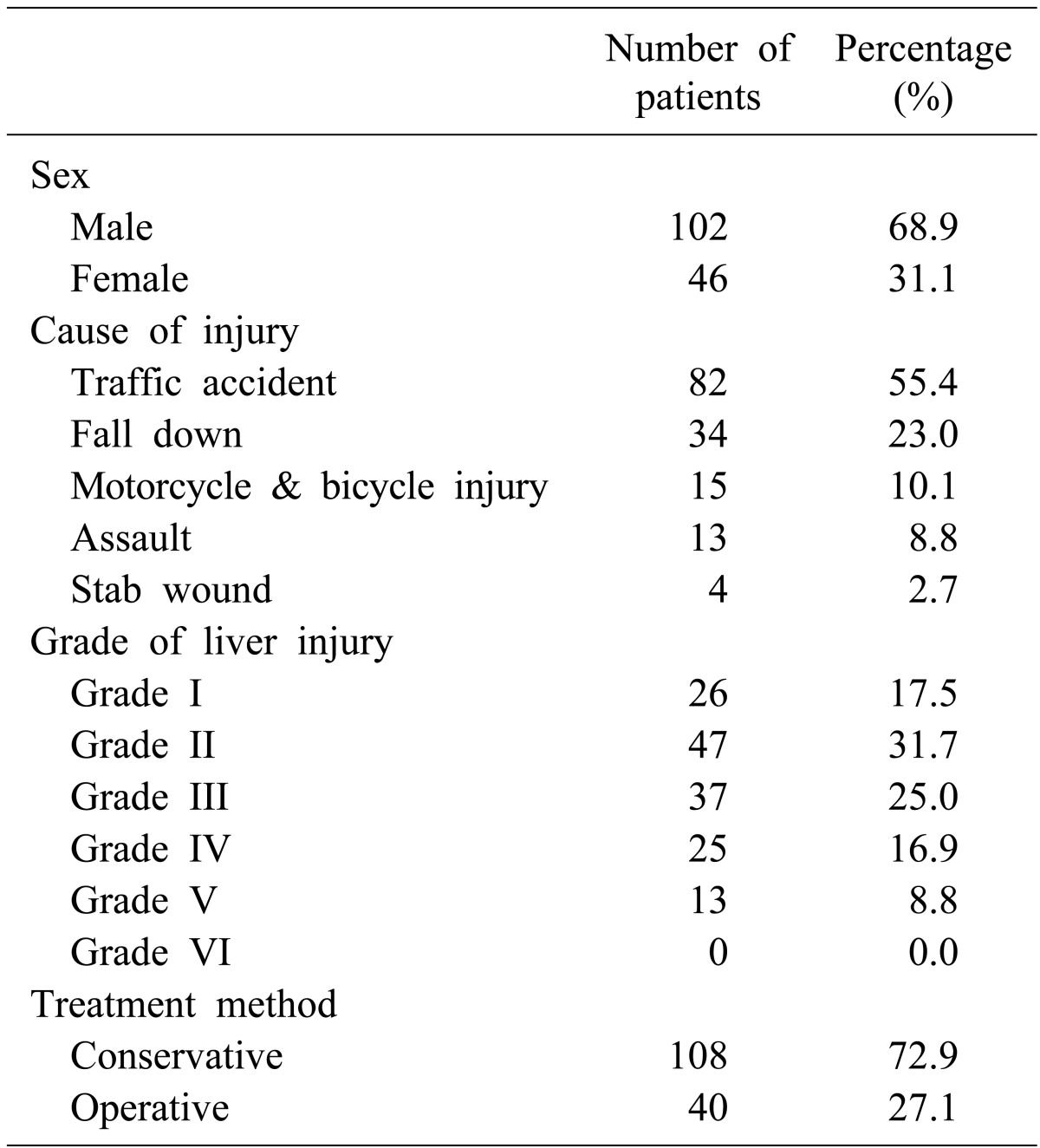
According to the 148 patient records evaluated, 108 (72.9%) patients were treated with NOM, and 40 (27.1%) underwent surgery. Patients treated with NOM had significantly fewer severe injuries as rated using the Revised Traumatic Injury Scale, Injury Severity Score, and Glasgow Coma Scale. Grade of liver injury and number of patients with extravasation of contrast dye on CT and hemoperitoneum were higher in the operative group than in the NOM group. There were significant differences between the two groups for: heart rate, respiratory rate, systolic blood pressure, and mean hemoglobin levels at admission and after 4 hours. The operative group experienced a significantly higher mortality than the NOM group.
Go to : 
Because of its size and anatomical position, the liver is often injured when abdominal trauma occurs. About 5% of admissions to emergency departments worldwide are because of hepatic trauma.1 There has been an increase in hepatic injury diagnosis because of the advances in diagnostic modalities, such as computed tomography (CT). These advances have also resulted in a change from surgical treatment to non-operative management (NOM) for select patients with hepatic injuries. A prospective study found that NOM is safe for hemodynamically stable patients with blunt hepatic injuries, regardless of the injury severity.1 In most trauma centers, NOM has become the standard therapeutic modality in hemodynamically stable patients because of its low complication rate. In the United States in recent decades, 80% to 90% of hepatic injuries are managed non-operatively.2
However, data including 35,510 cases of hepatic injuries from the American College of Surgeons' National Trauma Data Bank showed that, despite a strongly significant increase in the use of NOM for hepatic trauma, the mortality rates have remained unchanged.3 Moreover, excessive use of NOM for some high-grade liver injuries increased the short- and long-term morbidity, including biloma, biliary fistulae, early or late hemorrhage, false aneurysm, arteriovenous fistulae, hemobilia, liver abscess, and liver necrosis.45 Furthermore, no definitive guideline exists yet on which patients can be safely managed non-operatively.
Thus, this study aims to present data from patients with traumatic liver injury and to compare clinical findings and outcomes between the operative and NOM groups to determine factors that will aid in treatment choice.
Go to : 
The medical records were reviewed for 148 patients with traumatic liver injury who were admitted from April 2000 to July 2012 to Seoul St. Mary's Hospital in Seoul and St. Vincent's Hospital, Suwon City, South Korea. The ethical committees of the aforestated hospitals examined and approved this study. The exclusion criteria were: patients who were younger than 14 years, initially treated in other hospitals, or referred to other hospitals.
All patients were diagnosed using computed tomography (CT). Liver injury was classified according to the revised liver injury scale (6 grades) of the American Association for the Surgery of Trauma (AAST).6 The severity of the overall injury was calculated from hospital records using the Revised Trauma Scale (RTS), Injury Severity Score (ISS), and Glasgow Coma Scale (GCS).
In accordance with their hemodynamic stability, 108 patients (72.9%) were treated conservatively (NOM group), and 40 patients (27.1%) underwent surgery (operative group). This study compared the severity of liver injury, grade of injury, extravasation of dye on CT, presence of hemoperitoneum, other organ injury, initial vital signs (heart rate, respiratory rate, systolic blood pressure), laboratory results (hemoglobin, liver enzymes), amount of transfusion, and mortality between the operative group and NOM group. There was also a comparison of the hemodynamic stability according to grade of liver injury.
Differences between groups were tested using the chi-square test, Fisher's exact test, and Mann-Whitney test. SPSS V18.0 (IBM Corp; Chicago, Illinois, USA) was used throughout. The statistical significance was set at p-value <0.05.
Go to : 
In total, 148 patients were admitted for traumatic liver injury. The majority of patients (68.9%) were male. One-hundred eight (72.9%) patients were treated with NOM, and 40 (27.1%) patients were treated surgically (Table 1). There was no patient treated with an intervention, such as embolization.
Blunt liver injury (n=144, 97.3%) occurred more frequently than penetrating injury (n=4, 2.7%). The main causes of injury were traffic accidents (n=82, 55.4%), followed by an accidental fall (n=34, 23.0%). Fifteen patients (10.1%) were injured in motorcycle or bicycle accidents, and 13 (8.8%) had been assaulted. Stab wounds occurred in 4 (2.7%) patients.
A total 110 patients (74.3%) were low-grade liver injury (grades I, II, or III) and 38 patients (25.7%) were high-grade liver injury (grades IV or V), in accordance with the AAST scale. Not one of the patients were considered grade VI (unsalvageable). Grade II liver injuries (n=47, 31.7%) were the most frequent, followed by grade III (n=37, 25.0%), grade I (n=26, 17.5%), grade IV (n=25, 16.9%), and grade V (n=13, 8.8%).
The most commonly injured other abdominal organ was the kidney (n=16), and other associated injured abdominal organs were the spleen (n=13), pancreas (n=8), bowel (n=7), and adrenal gland (n=4).
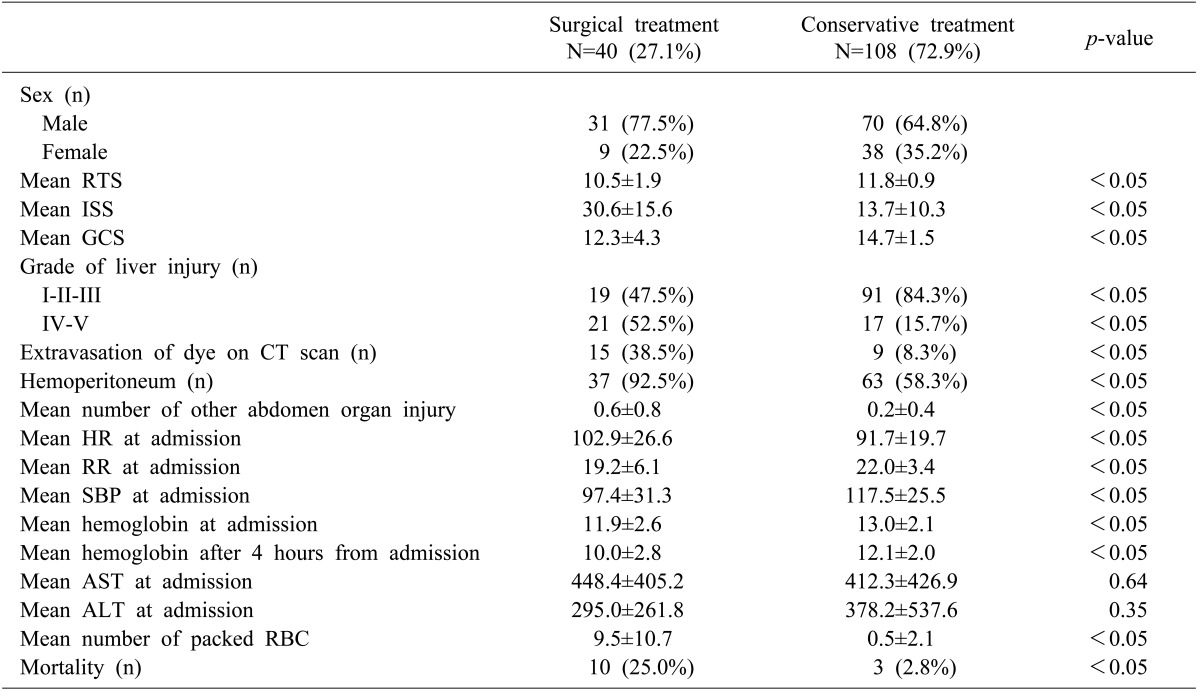
Here was a significant difference between the groups for: injury severity and grade, number of patients with extravasated contrast dye during CT or with hemoperitoneum, mean number of other abdomen organ injury, vital signs, hemoglobin levels, volume of transfusion, and mortality rate (Table 2). The NOM group showed a significantly lower ISS, higher RTS score, and higher GCS score than the operative group (p<0.05). The percentage of low-grade liver injury was significantly greater in the NOM group (84.3% vs. 47.5%). In contrast, the percentage of high-grade (grade IV and V) liver injury was greater in the operative group (n=21, 52.5%) versus the NOM group (n=17, 15.7%).
The number of patients with contrast dye extravasation during CT was significantly higher in the operative group (n=15, 38.5%) as compared with the NOM group (n=9, 8.3%). Hemoperitoneum was more commonly observed in the operative group (n=37, 92.5%, vs. n=63 58.3% for the NOM group). The mean number of other abdominal organ injuries was also significantly higher in the operative group (0.6 vs. 0.2, respectively).
The operative group had a higher average of initial heart rate and a lower average of initial respiratory rate and systolic blood pressure as compared with the NOM group.
In the laboratory findings, there were no differences between the two groups in initial aspartate aminotransferase and alanine aminotransferase levels. However, the mean hemoglobin at admission and 4 hours after were significantly lower in the operative group than in the NOM group (p<0.05). The change in hemoglobin level was also greater in the operative group. The mean number of packed red blood cells on admission was significantly greater in the operative group (9.5 vs. 0.5).
The mortality rate was significantly higher in the operative group than in the NOM group (25.0% vs. 2.8%). According to the medical records, deaths in the NOM group were caused by cardiac arrest in the emergency department (n=1), hypovolemic shock because of an external iliac artery laceration (n=1), and an external iliac vein laceration (n=1). These patients were being considered for surgery before they died. In the operative group, death was caused by hypovolemic shock (n=3), disseminated intravascular coagulopathy (n=3), multiorgan failure (n=2), cardiac arrest (n=1), and pulmonary embolism (n=1).
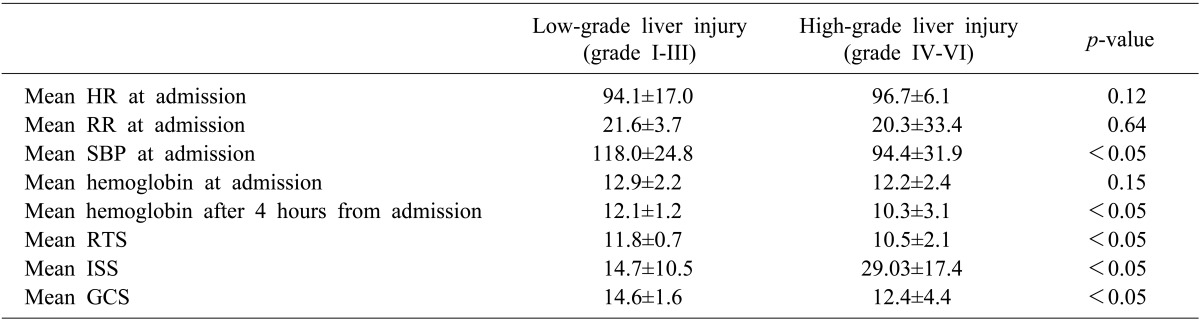
According to the grades of liver injury, there were no significant differences in the initial mean heart rate, mean respiratory rate, or mean hemoglobin (Table 3). In the low-grade group, the mean systolic blood pressure at admission was significantly higher than that of the high-grade group (118.0 mmHg vs. 94.4 mmHg, p<0.05). Furthermore, the mean hemoglobin after 4 hours was significantly higher in the low-grade group (12.1 g/dl vs. 10.3 g/dl, p<0.05). The mean RTS and GCS scores were higher (11.8 vs. 10.5, and 14.6 vs. 12.4, respectively, p<0.05) and the mean ISS was lower (14.7 vs. 29.03, p<0.05) in the low-grade group, as compared with the high-grade group.
Go to : 
The main cause of traumatic liver injury in the current study was traffic accidents (49.7%), which is similar to other published studies. In a multicenter study of 783 patients, 54% of traumatic liver injuries were caused by traffic accidents,7 while another study found that traffic accidents were responsible for 72% of traumatic liver injuries.8 Brammer et al.9 observed that 67% of liver injury patients were injured by traffic accidents.
Males were 69% of those with traumatic liver injuries in this study. A male predominance in this type of injury has been demonstrated in numerous other studies worldwide, including those conducted in the United Kingdom (79%),10 Scotland (76%),7 South Africa (81%),11 and the United States (65%).812
According to the injury grade, 70.9% of traumatic liver injuries in the current study were low-grade (I, II, or III), a finding similar to the results of previous studies. Pachter et al8. in their study described a predominance of grades I, II, or III injuries (80%). Scollay et al.7 found that most patients (69%) in Scotland with traumatic liver injury had AIS grade II injuries.
NOM is a safe and effective method in the management of hemodynamically stable patients with blunt hepatic injuries. The use of NOM in liver trauma has progressively increased: from 1969 to 1970, no patients were treated with NOM; from 1995 to 1999, however, the percentage had increased to 65%.13 In the current study, 72.9% of patients were treated with non-operatively from 2000 to 2012, which is similar to a study conducted in 2003.14
The current study showed significant differences in the grade of liver injury between the operative and NOM groups (p<0.05). In the NOM group, 84.3% of patients had low-grade injuries. Therefore, almost all patients with low-grade liver injuries in this study were treated non-operatively. In contrast, 15.7% of patients with high-grade injury were treated with NOM. There are reasons why high-grade liver injury is not well managed by NOM. First, high-grade injury is associated with hemodynamic instability. Second, patients with high-grade injury in the current study had a significantly lower mean systolic blood pressure at admission and reduced mean hemoglobin levels 4 hours after. Third, patients with high-grade injury may also have a severe injury to the brain, spleen, kidney, and other organs. Furthermore, a comparison of the ISS and RTS and GCS scores showed that a high-grade injury was associated with other severe injuries.
Most surgeons determine the treatment of traumatic liver injury according to a patient's hemodynamic status rather than the injury grade. The relationship between the liver injury grade and treatment choice remains controversial. In a study of 206 patients with liver injury, van der Wilden et al.15 found that liver injury grade was not significantly different between NOM failure versus success. Zago et al.16 showed no significant differences in liver injury grade between NOM and operative groups. However, Pachter et al.8 described that most cases of failed NOM occurred in patients with grades IV or V injuries. Furthermore, there are several studies showing that NOM in high-grade liver injuries may lead to significant morbidity and possible mortality because of liver-related complications.1718
In the current study, extravasation of contrast dye during CT and the number of patients with hemoperitoneum were significantly different in the two study groups (p<0.05). Fang et al.19 suggested that the presence of extravasation on CT is associated with a failure of NOM in initially hemodynamically stable patients with blunt liver trauma. The van der Wilden study of 206 patients with hepatic injury suggested a volume of 300 ml of free intraperitoneal fluid on CT as an independent risk factor for the failure of NOM.15 Thus, the extravasation of dye or presence of hemoperitoneum shown on CT could be factors considered for surgical interventional rather than NOM.
The operative group showed a higher mortality than the NOM group in the current study. According to the medical records, the most common cause of death was hypovolemic shock. A retrospective study of 44 patients with grade V blunt hepatic injuries reported that total intraoperative blood loss were the significant factors that determined operative mortality after blunt hepatic trauma.20 In this study, patients who died after surgery received a mean of 15.2 units of packed red blood cells, which is higher than the mean of 9.5 received in the first 24 hours. Most patients (80%) who failed surgery had grades IV and V liver injuries. Therefore, prompt resuscitation and appropriate surgical management are required to reduce mortality in patients with high-grade injury and significant blood loss.
There are currently no definitive guidelines for the treatment choice of traumatic liver injury. Asfar et al. suggested guidelines for the NOM of liver injury.21 The authors describe a continued need for blood transfusion exceeding 5 units, development of peritoneal signs, unstable vital signs despite resuscitation, and intrahepatic infections. Velmahos et al. identified 4 independent risk factors for NOM failure: presence of a splenic or renal injury, free fluids greater than 300 ml observed on CT, requirement for blood transfusion, and a high-grade liver injury.22 We suggest that when surgeons decide between surgery and NOM in patients with traumatic liver injury, considering the following factors will be helpful: hemodynamic stability; grade of liver injury; amount of blood loss; injury scales such as RTS, ISS, and GCS; and extravasation of contrast dye and hemoperitoneum in CT findings.
There were several limitations of this study. This study was retrospective study. We made decisions about the treatment in patients that were not in accordance with the standard clinical protocol. We evaluated only mortality but did not evaluate complications; thus, we could not assess the causes of NOM failure.
In this study, we compared the clinical features between NOM and surgical treatment of traumatic liver injury. There were significant differences between the two groups for: injury severity scores, grade of liver injury, extravasation of dye on CT, hemoperitoneum, other abdominal organ injuries, vital signs, hemoglobin levels, amounts of transfusion, and mortality rate. Thus, high-grade liver injury is associated with hemodynamic instability.
Considering the results of this study, we propose that hemodynamic stability and the following may be helpful when determining the treatment of traumatic liver injury: grade of liver injury, amount of blood loss, and injury scales scores, such as the RTS, ISS, and GCS,.
Go to : 
References
1. Croce MA, Fabian TC, Menke PG, Waddle-Smith L, Minard G, Kudsk KA, et al. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Ann Surg. 1995; 221:744–753. PMID: 7794078.

2. David Richardson J, Franklin GA, Lukan JK, Carrillo EH, Spain DA, Miller FB, et al. Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg. 2000; 232:324–330. PMID: 10973382.

3. Hurtuk M, Reed RL 2nd, Esposito TJ, Davis KA, Luchette FA. Trauma surgeons practice what they preach: The NTDB story on solid organ injury management. J Trauma. 2006; 61:243–254. PMID: 16917435.

4. Trunkey DD. Hepatic trauma: contemporary management. Surg Clin North Am. 2004; 84:437–450. PMID: 15062654.

5. Strong RW, Lynch SV, Wall DR, Liu CL. Anatomic resection for severe liver trauma. Surgery. 1998; 123:251–257. PMID: 9526515.

6. Tinkoff G, Esposito TJ, Reed J, Kilgo P, Fildes J, Pasquale M, et al. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg. 2008; 207:646–655. PMID: 18954775.

7. Scollay JM, Beard D, Smith R, McKeown D, Garden OJ, Parks R. Eleven years of liver trauma: the Scottish experience. World J Surg. 2005; 29:744–749. PMID: 15880277.

8. Pachter HL, Knudson MM, Esrig B, Ross S, Hoyt D, Cogbill T, et al. Status of nonoperative management of blunt hepatic injuries in 1995: a multicenter experience with 404 patients. J Trauma. 1996; 40:31–38. PMID: 8576995.
9. Brammer RD, Bramhall SR, Mirza DF, Mayer AD, McMaster P, Buckels JA. A 10-year experience of complex liver trauma. Br J Surg. 2002; 89:1532–1537. PMID: 12445061.

10. John TG, Greig JD, Johnstone AJ, Garden OJ. Liver trauma: a 10-year experience. Br J Surg. 1992; 79:1352–1356. PMID: 1486439.

11. Krige JE, Bornman PC, Terblanche J. Liver trauma in 446 patients. S Afr J Surg. 1997; 35:10–15. PMID: 9164149.
12. Fabian TC, Croce MA, Stanford GG, Payne LW, Mangiante EC, Voeller GR, et al. Factors affecting morbidity following hepatic trauma. A prospective analysis of 482 injuries. Ann Surg. 1991; 213:540–547. PMID: 2039284.
13. Lucas CE, Ledgerwood AM. Changing times and the treatment of liver injury. Am Surg. 2000; 66:337–341. PMID: 10776869.
14. Velmahos GC, Toutouzas K, Radin R, Chan L, Rhee P, Tillou A, et al. High success with nonoperative management of blunt hepatic trauma: the liver is a sturdy organ. Arch Surg. 2003; 138:475–480. PMID: 12742948.
15. van der Wilden GM, Velmahos GC, Joseph DK, Jacobs L, Debusk MG, Adams CA, et al. Successful nonoperative management of the most severe blunt renal injuries: a multicenter study of the research consortium of New England Centers for Trauma. JAMA Surg. 2013; 148:924–931. PMID: 23945834.
16. Zago TM, Pereira BM, Calderan TR, Hirano ES, Rizoli S, Fraga GP. Blunt hepatic trauma: comparison between surgical and nonoperative treatment. Rev Col Bras Cir. 2012; 39:307–313. PMID: 22936230.
17. Kozar RA, Moore JB, Niles SE, Holcomb JB, Moore EE, Cothren CC, et al. Complications of nonoperative management of high-grade blunt hepatic injuries. J Trauma. 2005; 59:1066–1071. PMID: 16385280.

18. Goldman R, Zilkoski M, Mullins R, Mayberry J, Deveney C, Trunkey D. Delayed celiotomy for the treatment of bile leak, compartment syndrome, and other hazards of nonoperative management of blunt liver injury. Am J Surg. 2003; 185:492–497. PMID: 12727573.

19. Fang JF, Wong YC, Lin BC, Hsu YP, Chen MF. The CT risk factors for the need of operative treatment in initially hemodynamically stable patients after blunt hepatic trauma. J Trauma. 2006; 61:547–553. PMID: 16966985.

20. Chen RJ, Fang JF, Lin BC, Hsu YP, Kao JL, Chen MF. Factors determining operative mortality of grade V blunt hepatic trauma. J Trauma. 2000; 49:886–891. PMID: 11086781.

21. Asfar S, Khoursheed M, Al-Saleh M, Alfawaz AA, Farghaly MM, Nur AM. Liver Trauma Registry Group. Management of liver trauma in Kuwait. Med Princ Pract. 2014; 23:160–166. PMID: 24457986.

22. Velmahos GC, Toutouzas KG, Radin R, Chan L, Demetriades D. Nonoperative treatment of blunt injury to solid abdominal organs: a prospective study. Arch Surg. 2003; 138:844–851. PMID: 12912742.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download