INTRODUCTION
Recent advances in ultrasonography contributed to the early detection of gallbladder (GB) cancer. Eighty percent of GB cancers are characteristically detected as polypoid lesions, and differential diagnosis is only carried out after pathologic examination.
1 The treatment of a polypoid lesion of the gallbladder (PLG) has been carried out in accordance with the "Gallbladder polyp practice recommendation" issued by the Korean Association of Hepato-Biliary-Pancreatic Surgery (KAHBPS). According to the recommendation, simple cholecystectomy is an adequate treatment for Tis and T1a lesions because there is no difference in survival rate with radical resection in early gallbladder cancer. On the other hand, GB cancer with T2 and above should be treated with radical resection.
2 Radiologic findings have some limitations in estimating GB cancer and depth of invasion through imaging findings. GB cancer is suspected preoperatively in only 30% of patients whereas the other 70% are discovered incidentally after following a simple cholecystectomy for other diseases such as GB stones and GB adenomyomatosis.
3,
4 It is difficult to determine the appropriate surgical approach for PLG preoperatively, especially when neoplastic polypoid lesions are suspected. In this study, we attempted to predict the progression of the disease by comparing the size of polypoid lesions, and we suggest that the size of the lesion would be a useful standard to determine an appropriate primary surgical approach for a PLG.
Go to :

METHODS
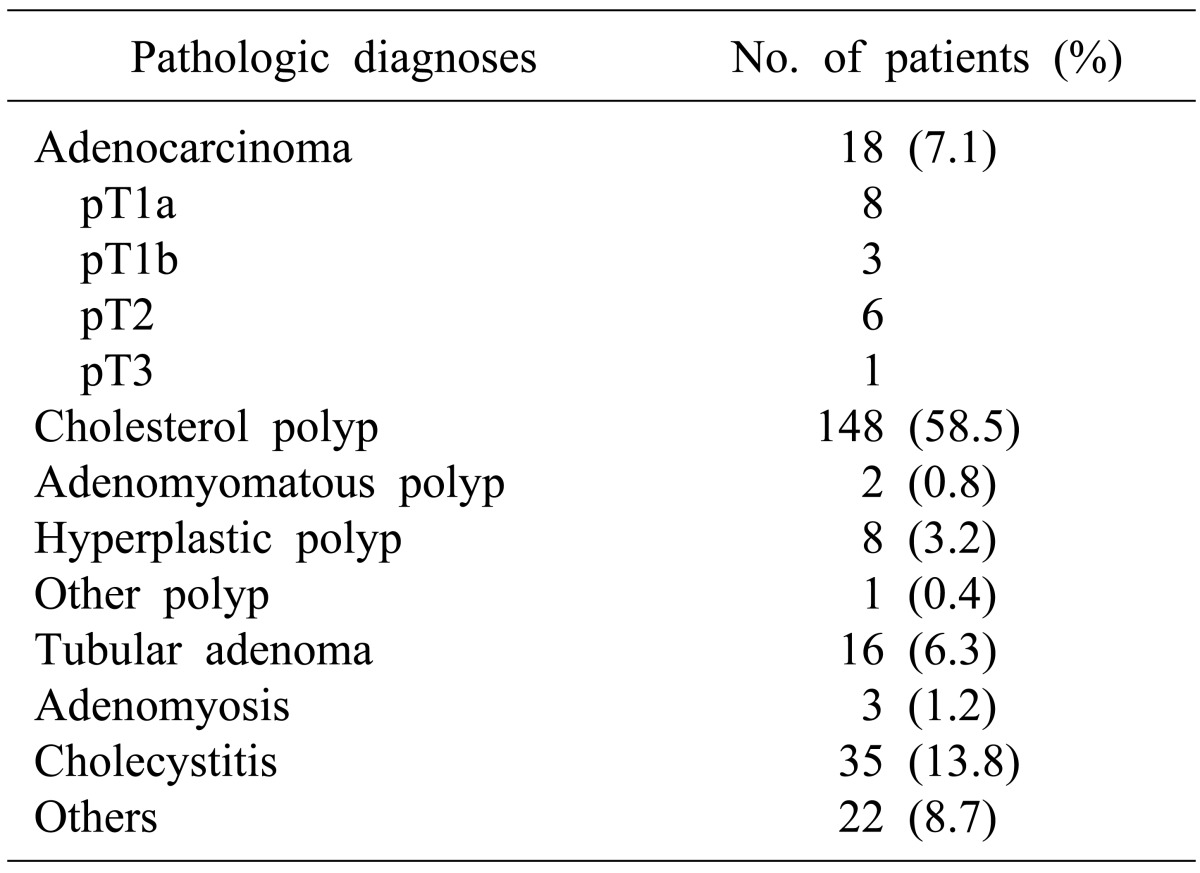
We obtained data on 253 patients with PLGs that had had a preoperative radiologic examination of the gallbladder and subsequently underwent cholecystectomy between January 2009 and December 2011 at our institution. We analyzed the sex, age, preoperative polypoid lesion size during radiologic examination, surgical method, tissue pathologic findings, T stages of 253 patients. The Mann-Whitney U test was used to evaluate the correlation between size, malignancy, and T stages. Receiver operating characteristic (ROC) curve analysis was also performed for correlation analysis.
Go to :

DISCUSSION
The prevalence of PLGs in healthy subjects varies from 0.004% to 13.8%.
5 In Korea, PLG prevalence was 6.1%.
6 PLGs were first defined pathologically by Christense and Ishak.
7 Generally, these lesions were categorized into benign and malignant groups. Benign lesions consist of neoplastic polyps (adenoma, hemangioma, lipoma), non-neoplastic polyps (cholesterol polyps, inflammatory polyps, hyperplastic polyps). On other hand, malignant lesions include adenocarcinoma, Squamous cell carcinoma, etc.
7,
8
Adenomas are benign growths in the wall of the GB and are diagnosed in 0.5% of all cholecystectomy cases. They are usually single lesion and pedunculated.
9 Most malignant polyps are adenocarcinoma. There are some studies that demonstrate the presence of adenomatous areas in carcinomas as well as the presence of malignant focus in gallbladder adenomas. Kozuka suggested the adenoma-carcinoma carcinogenic sequence.
10,
11
GB cancer is an aggressive malignancy and carries an extremely poor prognosis. The only chance of cure comes from early detection and curative surgery.
6 There are known risk factors that increase the likelihood of malignancy in a polypoid lesion, and these are size, number, morphologic type and growth rate of polyp, advancing age, and presence of gallstones.
12,
13 Larger poyp size increases the likelihood of malignancy.
14,
15 Terzi et al. reported the histopathologic characteristics of polypoid lesions in 100 patients who had cholecystectomy. Of the 74 patients with benign PLGs, only 11 (15%) had polyps larger than 10 mm. In contrast, 23 (88%) of the 26 malignant polyps were larger than 10 mm.
16 A study in Korea reported that polyps larger than 5mm had potential for malignancy and all polyps larger than 15 mm were malignant.
17 In these above-mentioned studies, larger polyp size suggests higher likelihood of malignancy. Generally, a patient who has a PLG larger than 10 mm would be recommended to undergo cholecystectomy.
14,
16
In patients with PLG larger than 10 mm, there is a dilemma in determining the primary surgical approach. Some patients received simple laparoscopic cholecystectomy, but other patients underwent radical cholecystectomy. According to KAHBP gGuidelines, simple and minimally invasive laparoscopic cholecystectomy is likely to provide an acceptable surgical outcome compared to that of radical surgery in patients who have early GB cancer and hardly suspected GB cancer. However, the depth of invasion can only be confirmed through pathologic examination. Frozen-section biopsy analysis can be helpful during operation, but there are errors in predicting the depth of the lesion in some cases. The larger size of polyp increases the likelihood of malignancy as well as increases the likelihood of advance. We focused on those points and studied how to predict early GB cancer preoperatively using a simple standard, such as polyp size.
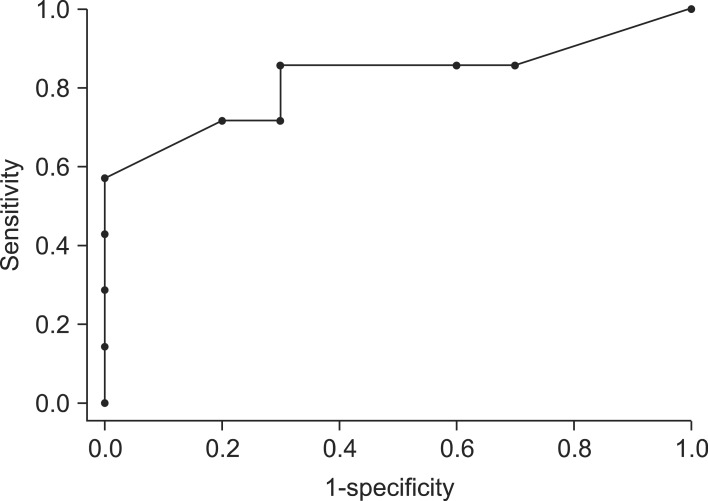
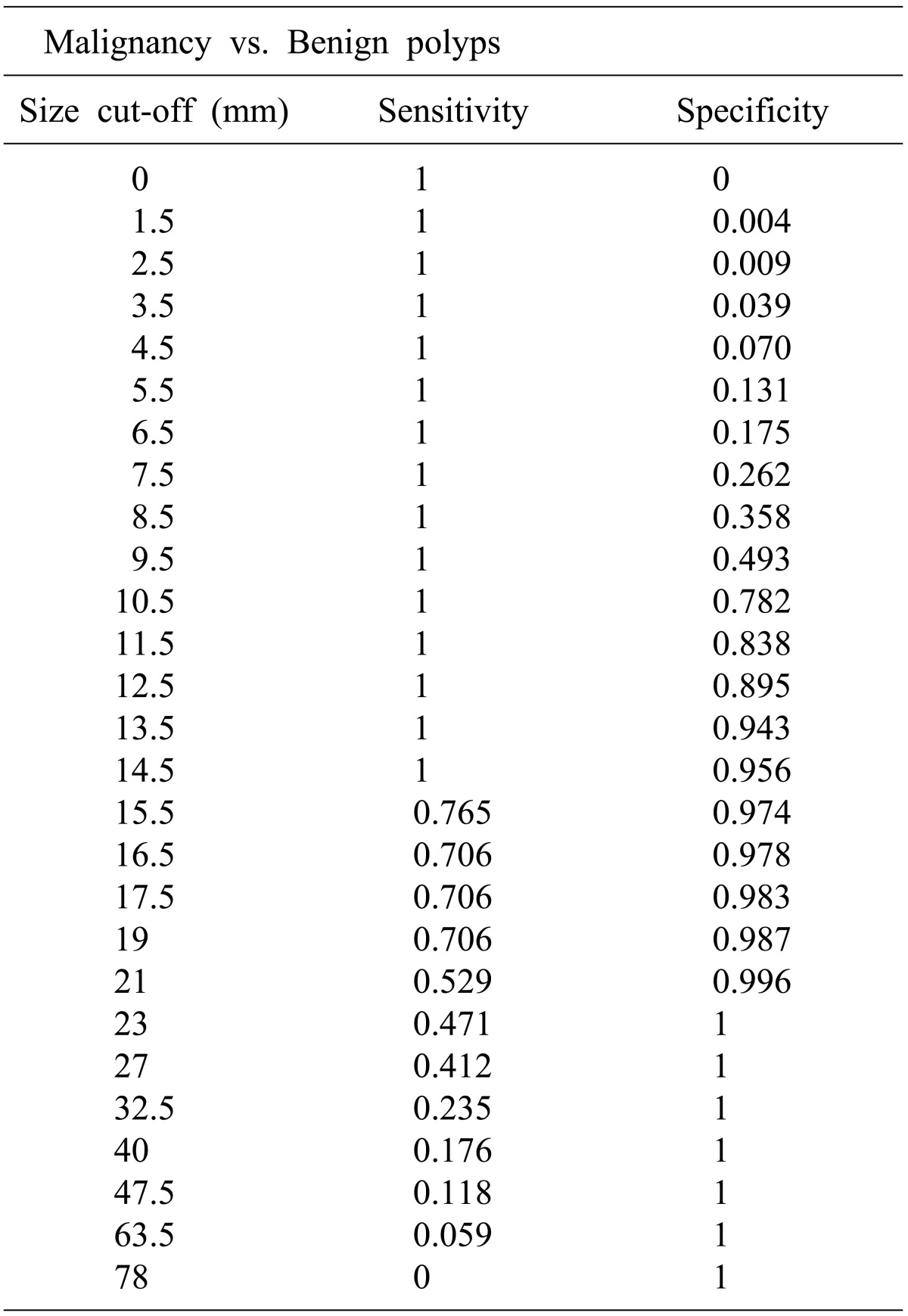
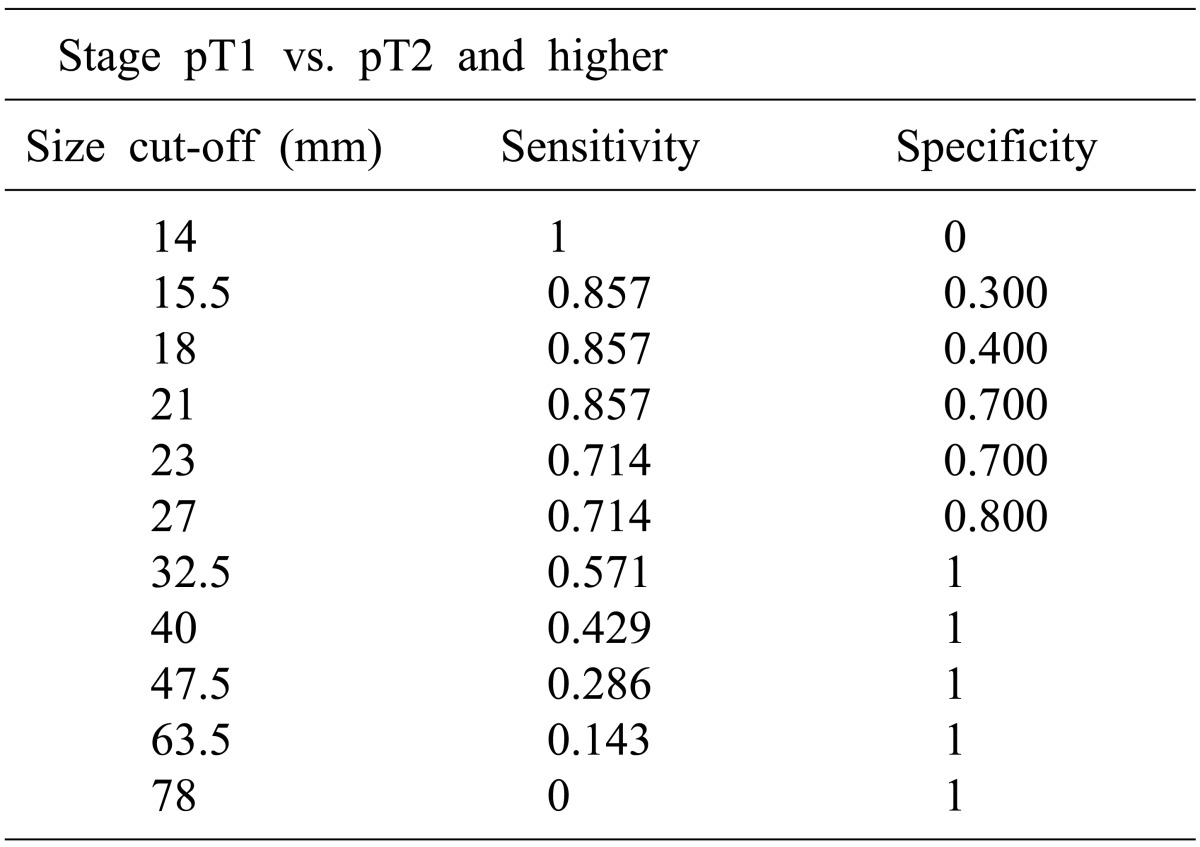
In this study, there is a significant difference in the average sizes of malignant (28.2±16.4 mm) and benign lesions (9.1±3.1 mm). The ROC curve shows 14.5 mm is the optimal point of predicting the malignancy. We identified there is statically significant difference in average sizes between T1 (20.5±5.8 mm) and T2 and higher (39.1±20.7 mm) GB cancers. We also presented that 27 mm would be the optimal point to predict above T2 cancer through ROC-curve. In patient with a PLG size larger than 14.5 mm but less than d less 27 mm, although the lesion is suspected as malignancy, the stage is considered as T1 cancer. Laparoscopic cholecystectomy can be performed as an appropriate primary surgical approach reasonably.
We attempted to figure out whether the simplest standard, size of polypoid lesions, can be an obvious criterion in predicting malignancy or invasiveness. This criterion using size is very simple, but cannot be a definite and only way to determine malignancy and invasiveness. Other factors also can affect the risk of malignancy. That might be limitation of this study. Lack of a higher number of patients involved in the study can be another limitation. Furthermore, a large multicenter study will be required to create safe and definite criteria to predict malignancy and invasiveness of PLGs.
Once a malignancy is diagnosed after laparoscopic cholecystectomy, potentially curative therapy should be considered.
2 The therapeutic surgical treatment of gallbladder cancer according to stage is supported by most surgeons.
18,
19,
20 Most surgeons agree that simple cholecystectomy is an adequate treatment for pTis and pT1a lesions, and that tumors stage pT2 and above should be treated by an additional radical operation. However, there is controversy regarding the management of T1b disease.
21,
22 In the present study, only 3 patients had T1b tumors and radical surgery was performed in only one case. All T2 and T2 and above tumor patients underwent radical surgery as a primary or second procedure following simple cholecystectomy.
In summary, the majority of PLGs are benign, and only a small portion thereof develops GB cancer. In the case of an early cancer, curative treatment can be achieved through simple and minimally invasive laparoscopic cholecystectomy. We attempted to predict early cancer within PLGs based on the simplest standard, size. We suggest 14.5 mm is the optimal point to predict malignancy of PLGs, and 27 mm is the optimal point to predict T2 cancer and above. Although there are some limitations, size can be a simple and easy way to evaluate PLGs.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download