This article has been
cited by other articles in ScienceCentral.
Abstract
Postoperative pancreatic fistula (POPF) combined with postoperative fluid collection, bleeding and abscess formation is one of the most critical morbidities after distal pancreatectomy or pancreaticoduodenectomy. Percutaneous catheter drainage has been commonly used for managing for the postoperative management of abnormal fluid collection. Removal of the catheter is rarely associated with occurrence of life-threatening complication such as serious liver damage. Herein, we report a case of unexpected fatal liver injury complicated by percutaneous catheter drainage treatment after distal pancreatosplenectomy in a patient with pancreatic cancer. We suggest that prudent decision for timing of catheter removal and meticulous care during procedure can reduce the possibility of major liver injury in patients with percutaneous transhepatic catheter drainage.
Go to :

Keywords: Liver injury, Complication, Percutaneous catheter drainage, Distal pancreatosplenectomy, Pancreatic cancer
INTRODUCTION
Pancreatic cancer is one of the most lethal malignant diseases of the gastrointestinal system and curative resection in pancreatic cancer is essential for long-term survival. With the advance of surgical techniques and perioperative management, conventional pancreatectomy, such as distal pancreatectomy or pancreaticoduodenectomy, is regarded as a safe and effective surgical procedure as a treatment of pancreatic cancer. With active clinical support from interventional radiology, most postoperative pancreatic fistula (POPF) combined with postoperative fluid collection, bleeding, and abscess formation can be managed in a conservative management.
1,
2 Percutaneous catheter drainage is a common procedure and helpful method for the postoperative management of infected or non-infected abnormal fluid that is collected in the peritoneal cavity.
3 However, we recently experienced an unexpected, life-threatening liver laceration immediately after removing a percutaneous drainage catheter from a patient who had a huge fluid collection following distal pancreatosplenectomy for left-sided pancreatic cancer, which became most embarrassing clinical situation to the patient as well as to the surgeons. This unexpected clinical event occurs very rarely, but it still needs to be considered its possibility in the daily practice of pancreatic surgery.
Go to :

CASE
A 74-year-old man presented with epigastric pain for 2 months and was diagnosed with pancreatic body cancer through diagnostic imaging studies. Computed tomography (CT), endoscopic ultrasonography (EUS), and magnetic resonance imaging (MRI) revealed a 3-cm-sized mass located on the body of the pancreas with suspicious perivascular infiltration around the celiac axis and superior mesenteric artery with distal pancreatic duct dilatation. Positron emission tomography (PET)-CT revealed no distant metastatic lesion. Several endoscopic trials had failed to obtain tissue samples for the pathologic conformation. Finally, exploratory laparotomy was performed for tissue diagnosis. No peritoneal metastasis was confirmed after the opening of peritoneum through the midline incision. In opening the lesser sac to evaluate for a pancreatic mass and celiac or SMA invasion, a hard pancreatic mass consistent with malignancy was observed on the body of the pancreas. Dissection of soft tissue around the celiac trunk was performed and the resected tissue was sent to a pathologic laboratory for prompt frozen section biopsy. This soft tissue sample was revealed to free of carcinoma. Anterior radical antegrade modular pancreatosplenectomy was performed without complication or event during operation.
The patient's postoperative recovery was uneventful and there was no remarkable postoperative complication. A routine postoperative follow-up imaging using abdomen CT scan was performed on postoperative day 7, in which there was free-fluid collection of 5.5×2.2 cm in size (
Fig. 1A) around the pancreatic resection margin. The pathologic examination confirmed pancreatic ductal adenocarcinoma with lymph node metastasis in 7 out of 19 lymph nodes. Both lymphovascular and perineural invasion were reported. The resection margin was free from carcinoma with a 2.5-cm safety margin, but the tangential margin near the superior mesenteric artery was very close to the malignant cells. The patient discharged on postoperative day 11 without any noticeable complication.
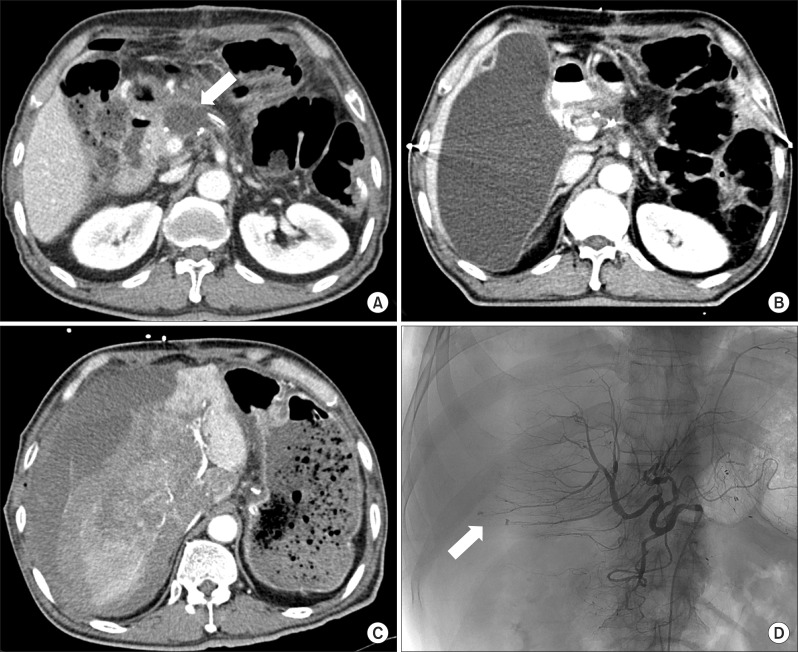 | Fig. 1Imaging sequences of percutaneous catheter drainage-related event in a 74-year-old man who underwent distal pancreatectomy with splenectomy for pancreatic cancer. CT scan taken on postoperative day 7 shows fluid collection (arrow) around pancreatic resection margin (A). One month after discharge, CT scan shows huge fluid collection in the subhepatic area (B). After removal of the percutaneous drainage catheter, CT scan shows extensive liver laceration with massive bleeding (C). Angiography shows extravasation of the contrast agent through the hepatic artery branches of Segment 5 and 6 (arrow) (D). 
|
One month after discharge, a follow-up abdomen CT scan was performed as baseline study for adjuvant chemoradiation therapy, and revealed that a large amount of fluid (18 cm in diameter was collected in the right subhepatic space (
Fig. 1B). Therefore, percutaneous drainage for this fluid collection in the subhepatic area was performed through a transhepatic approach. Amylase and lipase levels of the drained fluids were reportedly 3,921 U/L and 6,057 U/L, respectively. The amount of drained fluid and the levels of amylase/lipase decreased significantly 1 week after percutaneous drainage. The percutaneous drainage catheter was removed, as usual; however, after removal of the catheter, the patient presented with severe abdominal pain around the catheter removal site and systolic blood pressure fell to below 60 mmHg. After managing the shock, an emergency CT scan was performed, in which a large, newly developed hematoma with active extravasation of contrast material and severe tearing of the right liver parenchyma was noted, and total collapse of the intrahepatic inferior vena cava due to subcapsular hematoma (
Fig. 1C). Emergency hepatic angiography was performed to identify the bleeding focus and reveled active extravasations of contrast from branches of the right hepatic artery, however, there was no evidence of pseudo-aneurysm formation at the hepatic artery or other major arteries. Arterial embolization was performed promptly to stop hepatic bleeding. Liver enzymes had dramatically increased to more than 10,000 IU/L after embolization of the hepatic artery. Despite of vigorous supportive management in the intensive care unit, he died due to liver failure and cardiovascular complications at 4 days after the bleeding event.
Go to :

DISCUSSION
POPF is regarded as the most critical morbidity after pancreatectomy.
5 However, with the advance of interventional radiology, most cases of POPF can be successfully managed without an operative approach.
1,
2 Likewise, it is a common practice to recommend percutaneous catheter drainage for abnormal fluid collection following pancreatectomy.
3 Intraperitoneal fluid collection can be accessed by a direct percutaneous approach or a transhepatic approach. Approximately 15% of patients with percutaneous catheter drainage experienced several serious complications, such as infection, bleeding, and non-target catheterization or puncture.
4 However, it is extremely rare that catheter removal causes catastrophic liver damage leading to mortality, as shown in this case.
Two questions remain unanswered in this case patient: First, how did such a large amount of subhepatic, amylase-rich fluid collected? It is very unusual to find such a quantity of subhepatic fluid that has collected after a left-sided pancreatosplenectomy. In most cases of distal pancreatectomy, abnormal fluid usually collected around the resected pancreatic stump. Second, did catheter removal really cause this life-threatening laceration of hepatic parenchyma? The possibility of complication related to catheter removal seems to be low, but it is still possible. Nevertheless, the extent of liver damage was massive and aggressively destructive. Therefore we can assume another factor that contributed to worsening this catastrophic process. Amylase-rich fluid contacting the liver surface may, somehow, have affected the consistency of the liver parenchyma. There was no evidence of infection in the collected fluid and the cystic fluid looked clear and serous. There was no fever or leukocytosis. In addition, pancreatic fistula after distal pancreatectomy is known to be pure leakage of pro-enzyme. It is not clear whether non-activating pancreatic enzymes can really affect hepatic consistency. Finally, we can assume the possibility of expanding subcapsular hematoma compressing liver parenchyma from laceration originated from traumatic catheter removal. Extensive hemorrhage identified at mainly peripheral side of the liver and collapsed inferior vena cava indicated the possibility of expanding subcapsular hematoma of liver. Additionally, early timing of catheter removal before the maturation or granulation of punctured tract within the liver parenchyma could contribute to this extensive hemorrhagic event.
The transhepatic approach for percutaneous catheter drainage is regarded as a safe and effective procedure.
3 It is largely the responsibility of the interventional radiologist, however, to avoid the potential risk for a lethal complication, such as the one shown in our case. Direct percutaneous catheter drainage is more commonly recommended in managing abnormal fluid collection following conventional pancreatectomy. In current clinical practices where percutaneous catheter drainage is common, we suggest that pancreatic surgeons consider the possibility of lethal complication described herein, as it can cause unnecessary morbidity, mortality, and medico-legal problems. Prudent decision for timing of catheter removal and meticulous care during procedure can reduce the possibility of major liver injury in patients with percutaneous transhepatic catheter drainage.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download