This article has been
cited by other articles in ScienceCentral.
Abstract
Metastatic carcinoma that causes appendicitis is extremely rare. To our knowledge, metastatic cholangiocarcinoma in the appendix has been reported in only 1 case in the English literature. We report herein the case of an 87-year-old woman who presented with abdominal pain and jaundice. Advanced cholangiocellular carcinoma and a proximal appendiceal mass with appendicitis were detected on contrast-enhanced computed tomography and positron emission tomography/computed tomography. After elective laparoscopic appendectomy and wedge resection of the cecum, pathologic results revealed metastatic adenocarcinoma from extrahepatic cholangiocellular carcinoma in the appendix.
Go to :

Keywords: Cholagiocarcinoma, Appendicitis, Metastasis
INTRODUCTION
Acute appendicitis is one of the most common reasons for surgical abdomen. Obstruction of the appendiceal lumen with fecaliths or lymphoid hyperplasia is a well-known cause of appendicitis.
1 In extremely rare cases, metastatic cancerous lesions of the appendix can cause appendicitis. We herein present a case of appendicitis derived from metastatic extrahepatic cholangiocellular carcinoma.
Go to :

CASE
An 87-year-old female was admitted to the department of medicine with a chief complaint of abdominal pain with jaundice. She had a history of hypertension and type II diabetes mellitus for which she was treated at the local clinic with regular medications. Her vital signs were stable. Laboratory findings revealed a white blood cell count within normal range (5,680 cells/mm
3 with 74.7% segmented neutrophils), but C-reactive protein and the erythrocyte segmentation rate were elevated to 16.1 mg/L and 105 mm/hour, respectively. Liver function tests for aspirate aminotransferase, alanine aminotransferase, total bilirubin, direct bilirubin, alkaline phosphatase, and gamma-guanosinetriphospate were 284 IU/L, 403 IU/L, 9.1 mg/dl, 6.4 mg/dl, 540 IU/L, and 965 IU/L, respectively. Contrast-enhanced computed tomography (CT) revealed extrahepatic cholangiocellular carcinoma with direct hepatic invasion and lymph node metastasis in the para-aortic and peri-portal area. In addition, a dilated appendiceal lumen caused by a soft tissue mass at the base of the appendix was detected (
Fig. 1). Positron emission tomography-computed tomography (PET-CT) scan revealed an approximately 4.3 cm hypermetabolic mass in segment 4 of the liver with biliary dilatation and hypermetabolic nodes in the portocaval and para-aortic areas. Focal strong uptake in the proximal appendix suggested a malignant mass (
Fig. 2). The patient underwent an endoscopic retrograde cholangiopancreatography (ERCP)-guided biopsy of the common bile duct (CBD) and ultrasonography-guided biopsy of the metastatic hepatic mass. The result of the hepatic mass revealed adenocarcinoma; however, the results of the CBD revealed chronic non-specific inflammation.
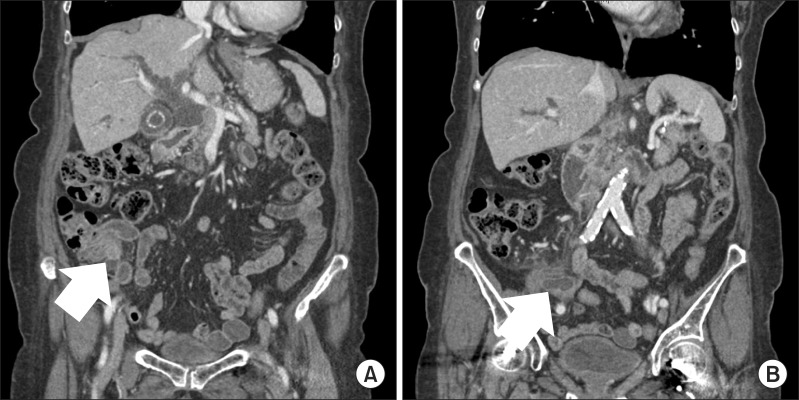 | Fig. 1Computed tomography finding: (A) A soft tissue mass of approximately 14 mm in diameter was located in the proximal appendix. (B) The appendiceal lumen was dilated to 13 mm with a thickened wall suggesting appendicitis. 
|
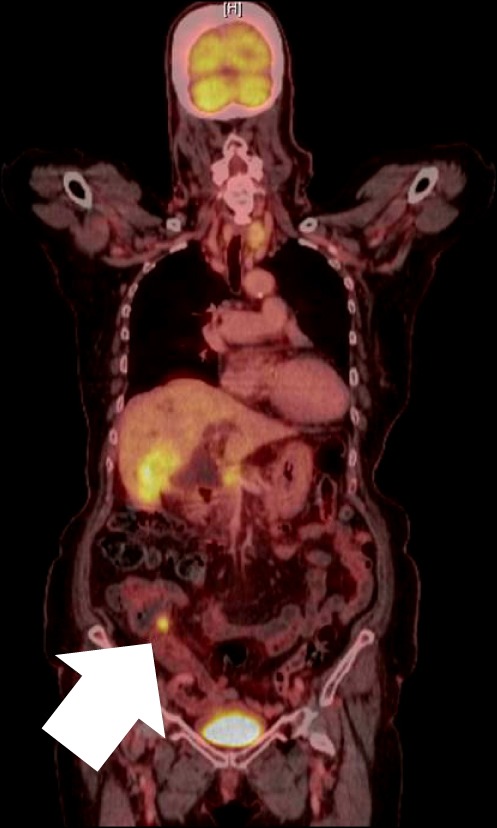 | Fig. 2Positron emission tomograhy finding: hypermetabolic mass was detected in the proximal appendix. 
|
The patient complained of diffuse abdominal pain in the right lower quadrant. Although the rebound tenderness was not severe, we were concerned that the degree of appendicitis might intensify as the metastatic mass in the appendix grew. Therefore, we decided to perform an elective laparoscopic appendectomy before the start of palliative chemotherapy. Surgery revealed a dilated appendix with minimal inflammation. A mass-like lesion was detected between the proximal appendix and the cecum base (
Fig. 3). For safe and complete resection, appendectomy with wedge resection of the cecum was performed. The pathologic report indicated metastatic adenocarcinoma involving the subserosa and proper appendiceal muscle involving the serosa through the mucosa of the cecum (
Fig. 4). Immunohistochemical staining revealed positive expression of cytokeratin 7 (CK7) but negative expression of cytokeratin 20 (CK20) and Cdx 2 in tumor cells, which was suggestive of adenocarcinoma of upper gastrointestinal or pancreaticobiliary origin (
Fig. 5).
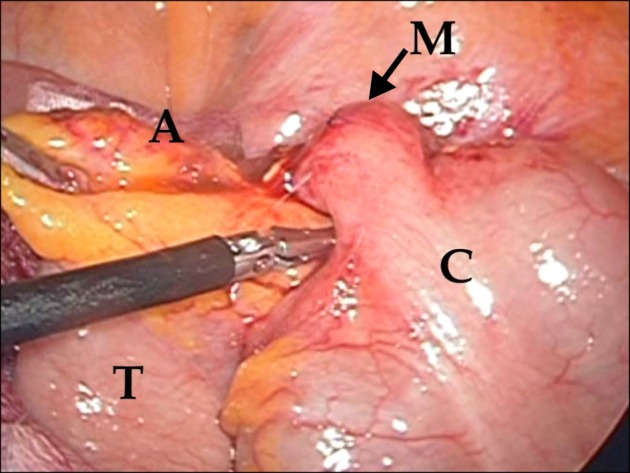 | Fig. 3Operative findings: A soft tissue mass was observed in the proximal appendix. A, appendix with inflammation; M, mass at the proximal appendix; C, cecum; T, terminal ileum. 
|
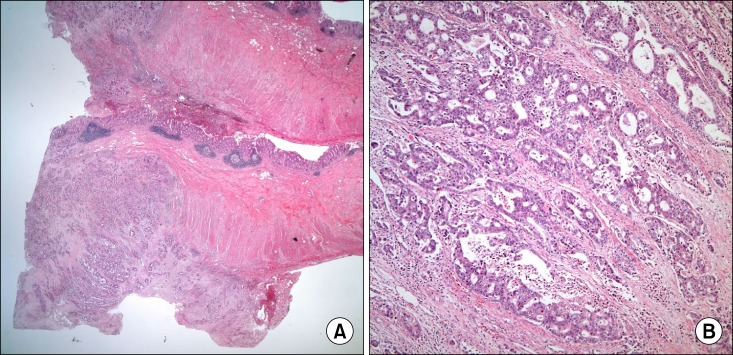 | Fig. 4Gross and microscopic findings of the surgical specimen: (A) The section shows multiple tumors infiltrating the appendiceal serosa through the submucosa (H&E, ×12.5). (B) The cancer cells are moderately differentiated adenocarcinoma (H&E, ×100). 
|
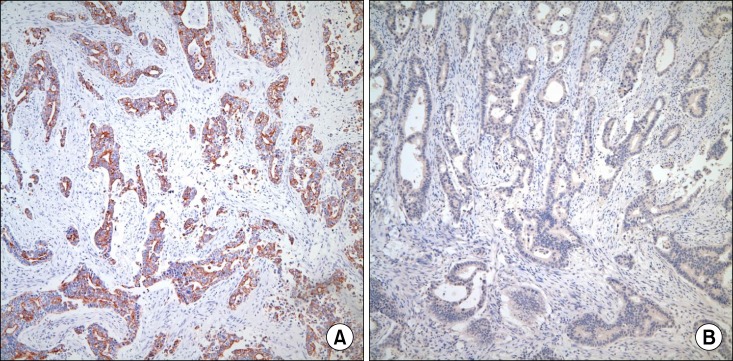 | Fig. 5Immunohistochemical staining of the surgical specimen: Immunohistochemical staining revealed positive expression of cytokeratin 7. (A) but negative expression of Cdx 2. (B) in the tumor cells (×100). 
|
The patient recovered from surgery uneventfully. She later underwent a self-expandable metallic stent insertion in the biliary tract to relieve bile duct obstruction; however, she refused to undergo palliative chemotherapy. Follow-up CT after three months of laparoscopic appendectomy revealed more progressive disease with peritoneal carcinomatosis.
Go to :

DISCUSSION
Luminal obstruction of the appendix caused by fecaliths or lymphoid hyperplasia is a major cause of appendicitis. Unusual factors such as parasites, endometriosis, bacteria, and primary and metastatic tumors can also cause appendicitis in rare cases.
1 Primary appendiceal cancer is rare and is found in only 0.9-1.4% of all appendectomy specimens. In addition, metastatic appendiceal cancer is much more rare.
2,
3 In a recent literature review, only seven patients were found to have metastatic appendiceal cancer among 80,698 patients.
1 The primary sites of metastatic appendiceal carcinomas were the lung, breast, cervix, prostate, stomach, liver, nasopharynx, mediastinum, and pancreas.
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13 As far as we know, primary cholangiocellular carcinoma very rarely metastasizes to the appendix.
14
Without imaging studies for patients with suspected appendicitis, it is very difficult to diagnose primary or metastatic carcinoma of the appendix.
2,
6,
7,
8,
9,
10,
11 As imaging modalities are more frequently used, appendiceal masses are found before surgery more commonly, sometimes with unusual characteristics.
3,
4,
5,
13 In a recent report of a case of appendiceal metastasis from a hepatocellular carcinoma, CT scan was useful in the diagnosis of the metastatic lesion. It showed a hypervascular area on arterial phase and a hypo-dense area on delayed phase imaging, two imaging features that are similar to those observed in primary hepatocellular carcinoma.
3 In our case, CT scan revealed an appendiceal mass with a dilated appendix and PET-CT revealed focal uptake of 18-FDG in the appendix base, which strongly suggested a malignant lesion. Although those examinations could not distinguish primary appendiceal carcinoma from a metastatic lesion of the appendix, they were helpful in the decision to perform an elective surgery.
It is known that cancer cells spread to other sites via the lymphatics, a hematogenous route, or direct invasion. The common metastatic sites of cholangiocellular carcinomas are regional lymph nodes and adjacent organs; distant metastasis is uncommon.
15 In particular, appendiceal metastasis from cholangiocellular carcinoma is an extremely rare event. It has been suggested that metastatic appendiceal carcinoma usually begins as serosal implants, which encroach progressively on the lumen.
6,
7 In this case, the pathologic results revealed that metastatic adenocarcinoma was involved through the serosa to the mucosa of the cecum. In addition, although there was no peritoneal seeding in the operative field, peritoneal carcinomatosis was confirmed by follow-up CT images three months after surgery. Thus, we think that serosal implantation with encroachment might be an adequate theory to explain the route of metastasis in the present case.
In conclusion, although appendicitis caused by metastasis to the appendix from other primary cancer sites is extremely rare, physicians should be more cautious in recognizing the possibility when patients complain of right lower quadrant abdominal pain. CT or PET-CT could be useful tools in detecting extraordinary appendicitis caused by metastatic extrahepatic cholangiocellular carcinoma.
Go to :


