Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, with high frequency rates in Asia. Many of the patients have unresectable disease at the time of diagnosis, and early detection and surgical resection is the best hope for survival. But, if HCC is presenting as an extrahepatic mass, the diagnosis is difficult. Herein, we report a case of primary HCC masquerading as a pelvic mass. A 74-year-old woman was admitted to our hospital due to a palpable mass in the lower abdomen. CT scan detected an approximately 15.0×13.4×11.4 cm-sized multilobulated homogeneous enhancing mass in the right adnexa. Operative findings showed that the pelvic mass originated from the liver. We performed hepatic wedge resection. Permanent histopathologic report revealed primary HCC. Exophytic-growing hepatocellular carcinoma should be carefully diagnosed.
Go to : 
Hepatocellular carcinoma (HCC) is a hyperendemic disease in Korea. Age-adjusted incidence was 24.5 per 100,000 population and 40.2 per year in males according to the Central Cancer Registry in Korea.1 The incidence is high compared to that in other countries. Mortality rate of HCC is second to that of lung cancer in males, and it is the fourth highest among females in Korea.1 But, pedunculated HCC (P-HCC) is a very unusual form of HCC. Horie et al. reported an incidence of P-HCC of 0.24-3.0% among all HCCs in Japan.2 Other abdominal tumors mimic P-HCC, and therefore, differential diagnosis is usually very difficult. In addition, diagnosis is delayed due to nonspecific symptoms and clinical features. Therefore, the resection rate of P-HCC is high, but the prognosis is poor due to recurrence and metastasis to the peritoneum, adrenal glands, and spleen.3,4,5 In our case also, it was difficult to diagnose P-HCC because it was mistaken for a pelvic mass. We describe a case of primary HCC that was detected incidentally during an obstetric operation.
Go to : 
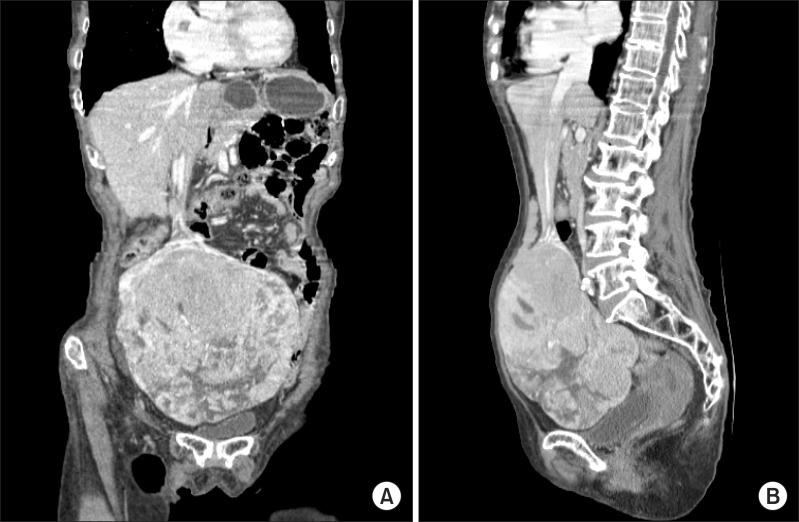
A 74-year-old woman was admitted to our hospital 3 years ago because of a palpable mass in the lower abdomen. Other symptoms such as nausea, vomiting, and abdominal pain were present. She also complained of vaginal discharge without bleeding. She did not have any other medical and surgical history. She had no history of blood transfusion or alcohol abuse. There was no family history of HCC. On physical examination, a huge palpable movable mass was detected in the lower abdomen, and the abdomen was slightly tender and distended. Blood tests demonstrated decreased hemoglobin/hematocrit, normal while blood cell and platelet count (10.4 g/dl/31.6%, 7,140/mm3 and 309,000/mm3, respectively). Other laboratory findings showed mild hyperbilirubinemia, serum total bilirubin 1.4 mg/dl and slightly elevated liver enzymes, Aspartate transaminase/Alanine transaminze 57/45 IU/L, alkaline phosphatase 149 IU/L. Hepatitis B virus surface antigen (HBsAg) and hepatitis C virus antibody (HCV-Ab) were negative. Also, tumor markers such as alpha-fetoprotein (AFP), cancer antigen (CA)-125, CA19-9, β-hCG (human chorionic goandotropin), Carcinoembryonic antigen (CEA) were within their normal limits. The other findings were unremarkable. Transvaginal ultrasonography revealed a huge solid mass with internal low echogenicity in the upper portion of the uterus, and CT scans showed a multilobulated homogeneous enhancing mass between the right adnexa and the left hepatic lobe measuring 15.2×11.4 cm (Fig. 1).
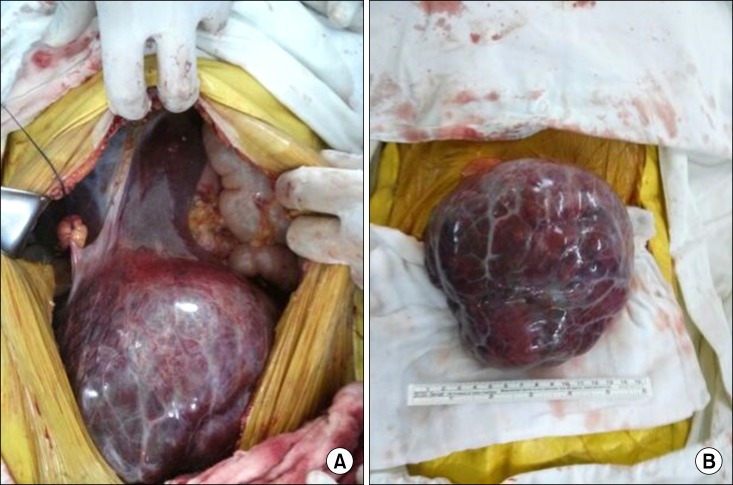
Hence, the initial impression was of a huge myoma uteri or right adnexal mass such as germ cell tumor (e.g. dysgerminoma, choriocarcinoma). Thus total abdominal hysterectomy with bilateral salpingo-oophorectomy was planned in the gynecologic department. During the operation, an exophytic mass with a thin peduncle originating from the left lateral hepatic lobe was observed. The mass was not infiltrating the uterus, the ovary and the other organs. Frozen-section biopsy was performed. The biopsy result was adenoma of uncertain origin, but carcinoma could not be excluded. Grossly, the tumor was fairly well-circumscribed and encapsulated. Remnant liver looked normal in color and consistency (Fig. 2). Therefore, we performed wedge resection of the hepatic mass leaving an adequate resection margin. She discharged on the tenth day after the operation without any complications. She was doing well for one year without adjuvant radiation and chemotherapy.
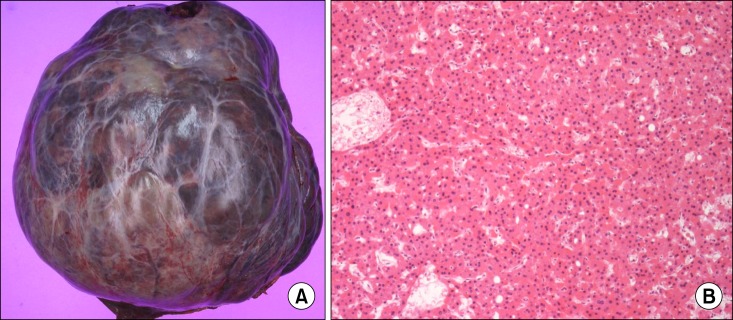
On pathologic examination, the mass was a 15×15 cm-sized nodular type, and the Edmondson and Steiner's histologic grade was III. Capsular invasion was present, but the other findings including vascular invasion were absent (Fig. 3).
Go to : 
Eggel classified the gross type of HCC into three types: nodular, diffuse, and massive.6 P-HCC is of the nodular type with exophytic growth. It was first described by Roux in 1897 as a carcinoma that protrudes from the liver with or without a pedicle.7 The first case of P-HCC was reported by Goldberg and Wallenstein in 1934, which showed a 1.5 cm-sized pedicle and it originated in the left inferior hepatic lobe.8 The incidence of P-HCC was reported to be 0.24%-3.0% of all HCCs in Japan.2 Of 432 surgical resected hepatocellular carcinomas, 18 (4.2%) were P-HCCs in a Taiwanese study, and the male to female ratio was 2:1 with a mean age of 47.8 years.9 According to their gross appearance, P-HCCs can be classified into pedunculated type with a pedicle as in our case, and pendunculated type without a pedicle and attached to the liver surface.10,11
The etiology of P-HCC is not clear. Mainly, P-HCC was mostly detected in the accessory lobe of the liver, and the accessory lobe is usually located on the right inferior hepatic lobe; however it can be located on the other lobe.12 P-HCC can also occur in the ectopic liver tissue such as triangular ligament, spleen, umbilical fossa, retroperitoneum, mesentery, and gallbladder. The preoperative diagnosis can be more difficult in such cases.2,13 When P-HCC develops in the right hepatic lobe with caudal growth?, which may show retroperitoneal extension, it is misdiagnosed as right adrenal tumor.5,14 Occasionally, it grows with direct invasion into the peritoneum and spleen. It is also misdiagnosed as a splenic or peritoneal mass.3,4 Similarly, it may invade the duodenum and mimic a duodenal gastrointestinal stromal tumor.15
Although the imaging modalities (CT and MRI) have improved, it is still difficult for a radiologist to determine the tumor origin. It can be misdiagnosed due to its location, and adherence to other organs. In our case, preoperative diagnosis was a right adnexal mass such as germ cell tumor or myoma uteri, but at the time operation, the mass was found to originate from the liver. Also, the laboratory findings were nonspecific. In a Japanese study, the positive rate of AFP, which is generally used as a tool for differential diagnosis between HCC and other abdominal tumors, was reported to be 68% in HCC and 66% in P-HCC, and there was no significant difference.16 But, Cunningham et al. reported that P-HCC is not related to hepatitis B virus infection and AFP.17 In our case, laboratory findings including hepatitis B virus status and tumor markers were also unremarkable.
The surgical procedure for P-HCC remains controversial. Some authors reported that anatomical hepatectomy was more suitable than partial resection. But others reported that if the growth of P-HCC was limited to the liver, wider resection margins could be obtained. Thus, P-HCC is more amenable to curative resection than conventional HCC. However, since the respectability rate according to the tumor location is high, hepatectomies for P-HCC do not always yield a survival benefit. The incidence of recurrence and disease-free survival is similar for these two entities.4,9
It has been reported that the prognosis is related to tumor size, capsular invasion, resection margin, and vascular invasion.9,18 Horie et al.19 reported that almost all P-HCCs show poorly differentiated characteristics using the Edmondson and Steiner's classification.20 But Yeh et al. reported that more than half of the pedunculated tumors were well differentiated in 18 out of 432 HCC patients. Additionally, the overall survival for patients with a large P-HCC (>5 cm) is better than the overall survival (54.5 months) of patients with non P-HCC type II. Especially, vascular invasion has been known to be associated with prognosis and recurrence of HCC.18 Vascular invasion is relatively low in the P-HCC type.13 In our case also, vascular invasion was not observed.
In conclusion, although the imaging modalities have improved, it is difficult to diagnose P-HCC due to its ambiguous origin. But if there is no vascular invasion, the prognosis is better than that in the other types. Also, it is easier to perform surgical resection of the P-HCC type than the other types. Therefore whenever possible, active treatment including surgical resection should be performed.
Go to : 
References
1. National Cancer Center. Korean national cancer registry annual report. http://www.ncc.re.kr.
2. Horie Y, Katoh S, Yoshida H, Imaoka T, Suou T, Hirayama C. Pedunculated hepatocellular carcinoma. Report of three cases and review of literature. Cancer. 1983; 51:746–751. PMID: 6295613.

3. Yan ML, Wang YD, Lai ZD, Tian YF, Chen HB, Qiu FN, et al. Pedunculated hepatocellular carcinoma and splenic metastasis. World J Gastroenterol. 2009; 15:5239–5241. PMID: 19891029.
4. Kurachi K, Suzuki S, Yokoi Y, Okumura T, Inaba K, Igarashi T, et al. A 5-year survivor after resection of peritoneal metastases from pedunculated-type hepatocellular carcinoma. J Gastroenterol. 2002; 37:571–574. PMID: 12162418.

5. Okuda K, Arakawa M, Kubo Y, Sakata K, Kage M, Iwamoto S, et al. Right-sided pedunculated hepatocellular carcinoma: a form of adrenal metastasis. Hepatology. 1998; 27:81–85. PMID: 9425921.

6. Eggel H. Über das primäre Carcinom der Leber. Beitr. Pathol Anat Allg Pathol. 1901; 30:506–604.
7. Roux C. Un cas de cancer primitif du foie avec pericholecystite calculeuse, perforation intestinale: hemostase hepatique. Rev Med Suisse Romande. 1897; 17:114–119.
8. Goldberg S, Wallerstein H. Primary massive liver cell carcinoma. Rev Gastroenterol. 1934; 1:305–313.
9. Yeh CN, Lee WC, Jeng LB, Chen MF. Pedunculated hepatocellular carcinoma: clinicopathologic study of 18 surgically resected cases. World J Surg. 2002; 26:1133–1138. PMID: 12209243.

10. Tzouliadis L, Hulin SJ, Shaw I, Rees M. Image of the month--pedunculated hepatocellular carcinoma. Arch Surg. 2007; 142:95. PMID: 17224508.
11. Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, et al. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983; 51:863–877. PMID: 6295617.

12. Omanik S, Jablonsky I. Pedunculated accessory hepatic lobe. Arch Surg. 1972; 105:792–794. PMID: 5081554.

13. Yeh CN, Lee WC, Jeng LB, Chen MF. Pedunculated hepatocellular carcinoma: clinicopathologic study of 18 surgically resected cases. World J Surg. 2002; 26:1133–1138. PMID: 12209243.

14. Kim KW, Auh YH, Chi HS, Lee SI. CT of retroperitoneal extension of hepatoma mimicking adrenal tumor. J Comput Assist Tomogr. 1993; 17:599–602. PMID: 8392525.

15. Kim HJ, Lee DH, Lim JW, Ko YT, Kim KW. Exophytic benign and malignant hepatic tumors: CT imaging Features. Korean J Radiol. 2008; 9:67–75. PMID: 18253078.

16. Hashimoto M, Degawa H, Sakamoto M. A case of extrahepatic growing hepatocellular carcinoma. Gastroenterol Surg. 1989; 12:1473–1477.
17. Cunningham PL, Nava H, Lopez C, Douglass HO Jr. Pedunculated primary hepatocellular carcinoma. J Surg Oncol. 1984; 27:260–267. PMID: 6094924.

18. Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996; 83:1219–1222. PMID: 8983610.

19. Horie Y, Shigoku A, Tanaka H, Tomie Y, Maeda N, Hoshino U, et al. Prognosis for pedunculated hepatocellular carcinoma. Oncology. 1999; 57:23–28. PMID: 10394121.

20. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954; 7:462–503. PMID: 13160935.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download