Abstract
Backgrounds/Aims
Conventional graft perfusion method using one small-caliber catheter takes a relatively long time for right liver graft perfusion, thus some modification is needed. In this study, we intended to assess the effectiveness of right liver graft perfusion methods through comparison of different infusion catheters.
Methods
The study consisted of two parts including one bench experiment to obtain data of hydraulic infusion and one clinical trial of 40 cases on graft perfusion with one- versus two-catheter infusion methods. These two graft infusion methods were compared in terms of the perfusion time and washing-out efficiency.
Results
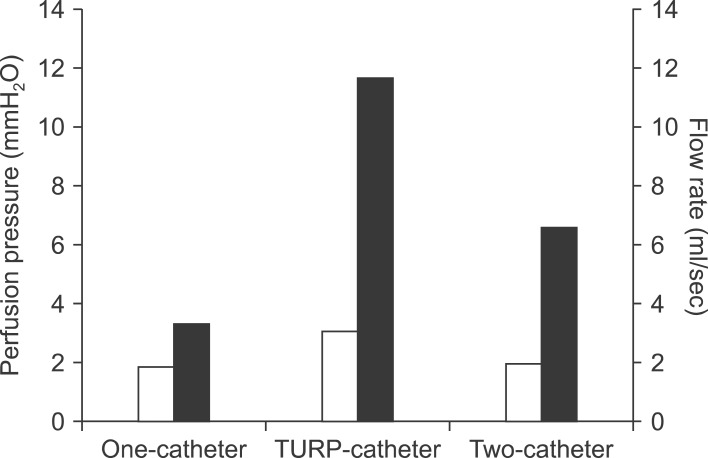
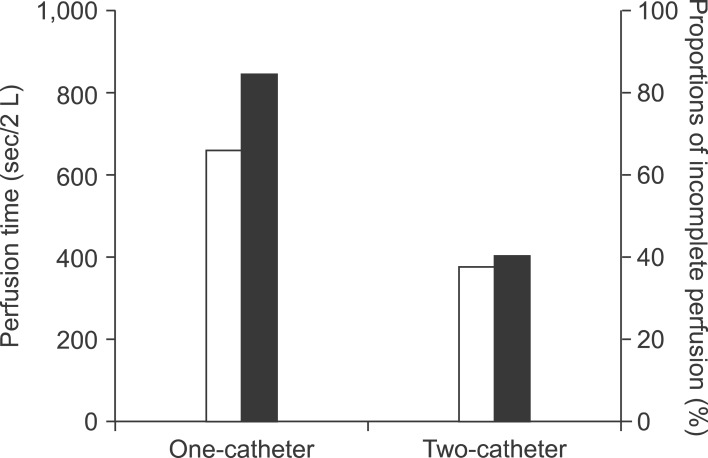
At bench experiment, the infusion flow rate and infusion pressure were 3.3 ml/sec and 1.9 cmH20 in one blood transfusion catheter group, and 11.7 ml/sec and 3.1 cmH20 in single transurethral resection of prostate irrigation catheter group, and 6.6 ml/sec and 2.0 cmH20 in two blood transfusion catheters group, respectively. In clinical trial with 40 right liver grafts, two-catheter group had a shorter graft portal perfusion time for the first 2 L of histidine-tryptophan-ketoglutarate (HTK) solution than the conventional one-catheter group (375±25 seconds vs. 662±34 seconds; p=0.001) and a lower rate of incomplete blood washing-out after the initial 2 L portal perfusion (40% vs. 85%; p=0.03).
Go to : 
While the surgical techniques for living-donor liver transplantation (LDLT) have developed greatly over the last 20 years, the methods of graft perfusion have little evolved. The graft perfusion method that was used during the early period of LDLT with left liver grafts was initial in-situ portal perfusion with cold lactate Ringer solution and then perfusate perfusion followed at the back table.1 Currently, such in-situ perfusion has been obsolete. Since right liver grafts have a short portal vein stump and brisk back bleeding, the in-situ perfusion step was not attempted and perfusate infusion started at the back table.2
The total graft washing-out time by using this classical perfusion method using 1.5 m-high hydraulic infusion usually depends on the size of an infusion catheter and the graft size. If the internal diameter of an infusion catheter is 5 mm, it takes 2-3 minutes for infusion of 1 L of low-viscosity perfusate solution (i.e., histidine-tryptophan-ketoglutarate [HTK] solution). In contrast, if a narrower catheter with an internal diameter of 3 mm is used, the infusion time increases to 5 minutes per 1 L of HTK solution.3 More viscous solution such as University of Wisconsin (UW) solution takes more time for hydraulic infusion than less viscous HTK solution. Thus, when using a small-caliber infusion catheter, some of the blood cells often remains in the liver graft because the hydraulic pressure-driven flow volume of perfusate is relatively too small to wash out blood cells effectively from the right liver grafts. Direct arterial irrigation is usually not performed because of the potential risk of intimal damage to the graft hepatic artery.
We have traditionally used a small-caliber infusion catheter for graft perfusion for a long time, but we recognized that our graft perfusion time was rather prolonged because the catheter has a small internal diameter which was originally designed for blood transfusion. Given that the normal portal perfusion in the human body is driven by low-pressure but high-volume splanchnic blood flow, we thought that it is necessary to speed up graft perfusion rate by using a larger-caliber catheter or two small-caliber catheters.
In this study, we intended to assess the effectiveness of right liver graft perfusion methods through comparison of different perfusion catheters.
Go to : 
The study consisted of two parts including one bench experiment to obtain data of hydraulic infusion and one clinical trial on graft perfusion.
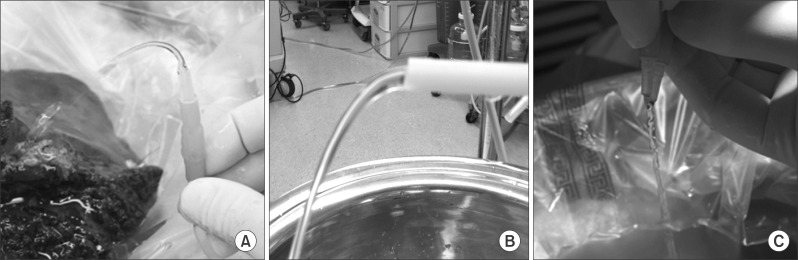
First, we measure the time to infuse 1 L of HTK solution by using catheters of different internal calibers under the height difference of 1.5 m at the back table environment. Perfusion pressure was assessed by measuring the height of flow after placing the catheter tip upward (Fig. 1).
Second, we prospectively measured the total perfusion time to infuse 2 L of HTK solution into the right liver grafts through using one or two infusion catheters. The completeness of graft blood washing-out was also assessed. This study was performed during 5 months from April and August 2011, and a total of 40 right liver grafts were allocated for one- and two-catheter groups.
Our conventional graft perfusion method consisted of gravitational dripping of HTK solution by using a standard blood transfusion catheter from a height of 1.5 m. For right liver grafts that weighed ≤600 g at the back table, a total of 2 L HTK solution was used for the initial portal perfusion. For grafts that were >600 g, 3 L was often used. Liver grafts weighing more than 800 g were excluded from this study to avoid bias. The graft bile duct was forcefully irrigated with a total of 30-60 ml HTK solution by using a syringe. We do not apply direct infusion into the graft hepatic artery. In addition to the prior portal perfusion using 2-3 L HTK solution, after back table procedure for middle hepatic vein reconstruction using interposition vessel grafts and hepatic vein venoplasty, another 0.5 L HTK solution was used for retrograde gentle irrigation by using a surgical spoid through the middle and right hepatic vein orifices. For ABO blood group-incompatible grafts, the amount of initial portal perfusion was increased to 3-4 L of HTK solution.
This study protocol was approved by the institutional review board for our institution. The continuous variables were expressed as mean±standard deviation and compared by using Student's t-test. The incidence variable was compared using Fisher's exact test. A p-value <0.05 was considered to be statistically significant.
Go to : 
The bench setting was gravitational dripping at the height difference of 1.5 m, which was the same as back table perfusion. Hydraulic infusion parameters are compared at Fig. 2.
When a standard blood transfusion catheter was used, the mean time to infuse 1 L HTK solution was 302±3 seconds after 5 trials. The mean height of upward flow was 1.9±0.3 cm. These results mean that the flow rate was 3.3 ml/sec and infusion pressure is 1.9 cmH20.
When a larger-sized catheter (transurethral resection of prostate [TURP] irrigation catheter) was used, the mean time to infuse 1 L HTK solution was 115±4 seconds after 5 trials. The mean height of upward flow was 3.1±0.4 cm. These results indicate that the flow rate was 11.7 ml/sec and infusion pressure is 3.1 cmH20.
We have used TURP irrigation catheters during deceased-donor liver recovery for last 20 years, but we did not use them during perfusion of living-donor liver graft. We have usually performed two separate perfusions when there were two graft portal vein orifices as in portal vein variation of type III (early branching of the right posterior sectoral portal vein). Thus, we determined to use conventional blood transfusion catheter for safety, and then set up the design of ongoing clinical trial to compare one-catheter versus two-catheter infusion.
When two standard blood transfusion catheters were used, the mean time to infuse 2 L HTK solution was 301±4 seconds after 5 trials. The mean height of upward flow was 2.0±0.3 cm. These results mean that the flow rate was 6.6 ml/sec and infusion pressure is 2.0 cmH20.
In the conventional one-catheter infusion group (n=20), the mean graft weight was 670±9 g and the graft-recipient weight ratio was 1.04±0.13. Graft portal perfusion required 662±34 seconds for the first 2 L of perfusate and 3-5 minutes for the next 0.5-1 L infusion. When gentle portal vein irrigation using a spoid was attempted after initial 2 L infusion, noticeable blood-tinged perfusate was drained in 17 of 20 right liver grafts (85%), thus additional perfusion of >0.5 L perfusate was performed to clear out the graft blood.
In the two-catheter infusion group (n=20), two catheters were concurrently inserted into the confluence of the right anterior and posterior portal vein stumps or separately inserted into these sectoral portal vein stumps. The mean graft weight was 681±78 g (p=0.46 vs. the one-catheter group) and the graft-recipient weight ratio was 1.05±0.89 (p=0.95 vs. the one-catheter group). Graft portal perfusion required 375±25 seconds for the first 2 L (p=0.001 vs. the one-catheter group) and 2-5 minutes for the next 0.5-1 L infusion. On additional test irrigation after initial 2 L infusion, blood-tinged perfusate drained from the portal vein in 8 of the 20 right liver grafts (40%) (p=0.03 vs. control group) (Fig. 3). In addition to the initial portal perfusion, a total of 0.5-1 L perfusate was needed to clear out the graft blood.
Go to : 
Conventional graft perfusion using a usual intravenous infusion or blood transfusion catheter required more than 10 minutes for the initial portal perfusion of 2 L perfusate. Since the actual perfusion pressure is usually 2-3 cmH20 under a gravitational dripping from a height of 1.5 m, the perfusion volume through a 2-3 mm-sized internal lumen is only around 3-4 ml/sec. Thus, unless a specially designed large-caliber catheter is used, the conventional method is regarded as a low-pressure low-volume infusion method. At the portal vein orifice of right liver grafts, there are two main sectoral vein orifices as well as a few small accessory vein openings. Considering such complex structure of intrahepatic portal vein anatomy, it is difficult to wash out the graft blood rapidly by using such a slow perfusion method. Indeed, in the present study, test portal irrigation revealed direct evidence of incomplete washing-out in 85% of the cases.
Historically, the conventional graft perfusion method for LDLT was developed for left liver grafts.1 Most left liver grafts weigh less than 500 g and have a longer first-order left portal vein stump, which indicates that such a low-pressure low-volume perfusion method can wash out the graft blood shortly. However, the present study showed that when the same conventional graft perfusion method was used for the right liver grafts, which are much larger and have a more complex portal vein anatomy than the left liver grafts, the perfusion time was prolonged and the washing-out efficiency was decreased. Since there is no reason to prolong the ischemic time at the back table, this observation led us to realize that some modified approaches were needed.
From the viewpoint of portal hemodynamics and hepatic perfusion, the native human portal perfusion condition must be the ideal. Since human portal perfusion is a kind of low-pressure high-volume system, a low-pressure high-volume perfusion method would be more suitable to wash out the right liver grafts than the conventional method. In fact, this perfusion method has been used for a long time for the portal perfusion of deceased-donor liver recovery. During graft recovery from deceased donors, a large-caliber TURP irrigation catheter is used for rapid perfusion through the superior or inferior mesenteric vein unless aorta only perfusion is performed. If a large-caliber catheter with an internal lumen of ≥5 mm is used, the flow volume will be increased to 10-20 ml/sec with a perfusion pressure of <10 cmH20. However, we worried about potential damage to the right liver graft because we had nearly no experience on it yet. Based on the hemodynamics data obtained from the bench experiment, the TURP irrigation catheter appears to be more adequate than the conventional transfusion set for bench perfusion of right liver grafts. In fact, TURP tubes have been used in a small number of major LDLT centers worldwide. From the viewpoint of safety, we attempted two-catheter infusion rather than single large-catheter infusion.
The potential demerits of incomplete graft washing include the risk of adverse immunological responses and unnecessary sensitization to various blood cells that result in problems such as graft-versus-host disease.4,5,6 At present, it is recommended that the graft blood should be completely washed out if possible, especially in ABO blood group-incompatible LDLT.
The merits of our two-catheter infusion method include a shorter perfusion time and more efficient graft blood washing-out. The perfusion pressure is actually not different between one- and two-catheter methods and is much lower than the normal range of portal pressure, thus there is no risk of portal hyperperfusion-associated sinusoidal injury.7,8
In conclusion, the two-catheter infusion method appears to be more effective than the conventional one-catheter infusion method for right liver graft perfusion at the back table. Large size of right liver grafts seems to be its good indication.
Go to : 
References
1. Ozawa K, Uemoto S, Tanaka K, Kumada K, Yamaoka Y, Kobayashi N, et al. An appraisal of pediatric liver transplantation from living relatives. Initial clinical experiences in 20 pediatric liver transplantations from living relatives as donors. Ann Surg. 1992; 216:547–553. PMID: 1280074.

2. Hwang S, Lee SG, Kim KH, Park KM, Ahn CS, Moon DB, et al. Correlation of blood-free graft weight and volumetric graft volume by an analysis of blood content in living donor liver grafts. Transplant Proc. 2002; 34:3293–3294. PMID: 12493450.

3. Testa G, Malagó M, Nadalin S, Treptow B, Paul A, Frilling A, et al. Histidine-tryptophan-ketoglutarate versus University of Wisconsin solution in living donor liver transplantation: results of a prospective study. Liver Transpl. 2003; 9:822–826. PMID: 12884194.

4. Sato Y, Watanabe H, Ichida T, Yamamoto S, Nakatsuka H, Oya H, et al. Wall shear stress and intrahepatic leukocytes of graft in living related donor liver transplantation. Hepatogastroenterology. 2004; 51:329–333. PMID: 15086151.
5. Hajeer AH, Issa S, Alaskar A, Abdullah K, Awad M, Tbakhi A, et al. Neutrophils and lymphoid chimerism after adult living-related liver transplantation from a homozygous donor. Transplant Proc. 2005; 37:4386–4388. PMID: 16387127.

6. Soejima Y, Shimada M, Suehiro T, Hiroshige S, Gondo H, Takami A, et al. Graft-versus-host disease following living donor liver transplantation. Liver Transpl. 2004; 10:460–464. PMID: 15004778.

7. Man K, Fan ST, Lo CM, Liu CL, Fung PC, Liang TB, et al. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003; 237:256–264. PMID: 12560784.
8. Puhl G, Schaser KD, Pust D, Köhler K, Vollmar B, Menger MD, et al. Initial hepatic microcirculation correlates with early graft function in human orthotopic liver transplantation. Liver Transpl. 2005; 11:555–563. PMID: 15838880.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download