Abstract
Backgrounds/Aims
Metastatic cancer of pancreas is rarely resectable. Pancreaticoduodenectomy carries high risks of morbidities and mortalities that it is rarely performed for metastatic cancer. In this study, the clinical features and outcomes of metastatic cancer of pancreas after pancreaticoduodenectomy were reviewed and analyzed.
Methods
We retrospectively reviewed patients who underwent pancreaticoduodectomy from January 2000 to December 2012 in Samsung Medical Center. A total of 1045 patients were enrolled in this study. Inclusion criteria were patients who had metachronous lesions with tumors histologically confirmed as metastatic cancer. However, patients with tumors directly invaded pancreas head, bile duct, and duodenum were excluded from this study. Finally, a total of 12 patients who underwent pancreaticoduodenectomy due to metastatic cancer were used in this study. Clinicopathologic features and perioperative data of these 12 patients were retrospectively reviewed.
Results
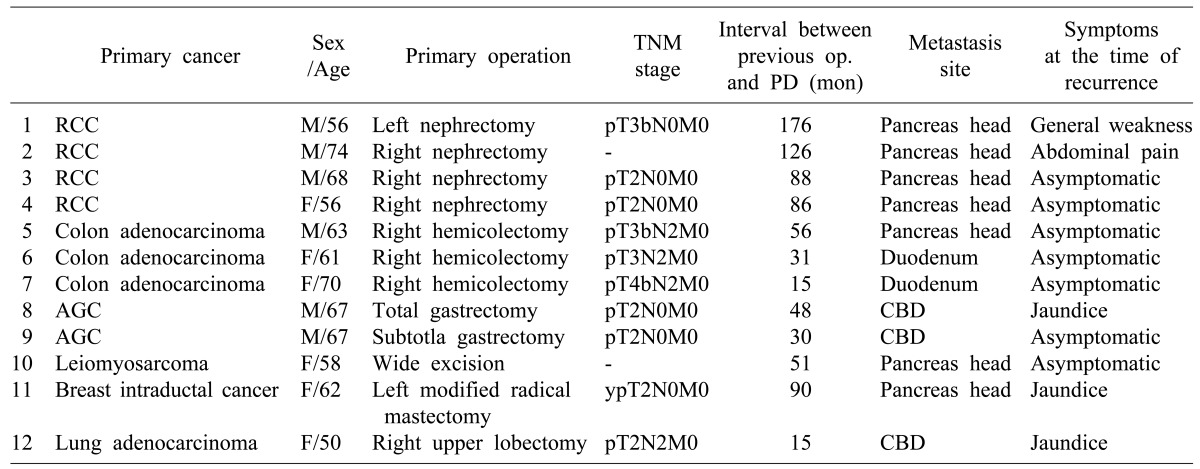
The 12 patients included 6 females and 6 males who had metastatic lesions at pancreas head, duodenum 2nd-3rd portion, and distal common bile duct. The mean age of patients was 62.7 years old at the time of pancreaticoduodenectomy. The interval between the time of the first operation for primary cancer and pancreaticoduodenectomy was 67.7 months. The mean survival time after pancreaticoduodectomy was 38.6 months (range, 12 to 119 months). There was no fatal complication after the surgery.
Conclusions
Pancreaticoduodenectomy is becoming a safer procedure with less complication compared to the past. Patients with recurrent metastatic cancer should be considered for metastectomy if tumors are resectable. Pancreaticoduodenectomy should be considered as one main treatment for patients with recurrent metastatic cancer to offer a chance of long-term survival in selected patients.
Recently, the number of pancreaticoduodenectomy has increased because early surgical treatment of patients has become possible due to the development in diagnostic technologies for cancer. The development of medical technologies and equipment as well as accumulated experience have gradually reduced the incidence of complications and mortality associated with pancreaticoduodenectomy.1,2 However, in many studies about surgical treatment of metastatic cancer at pancreatic head, bile duct, and ampulla of Vater, prognosis and the survival rates are poor and controversial. Renal cell carcinoma and advanced gastric, lung, and breast cancer are known to be primary cancers that are readily to metastasize to common bile duct and pancreas. In particular, surgical treatment of many patients with metastatic cancer from these primary cancers is impossible due to their aggressive characteristics. Recently, regular follow-up and development in the diagnosis of metastasis and recurrences after treatment of primary tumor (surgery, chemotherapy or radiation therapy) have made it possible to diagnose any recurrence or metastasis of the cancer early. Consequently, the resectability of these secondary tumors is increasing along with surgical treatment improvements.3,4,5
The objectives of this study were to examine patients requiring pancreaticoduodenectomy of peri-ampulla of Vater area metastasis (pancreatic head, common bile duct, duodenum, ampulla of Vater) from primary tumor and to study the indications for surgery, survival rate, and prognosis.
Of 1,045 patients who underwent pancreaticoduodenectomy from January 2000 to December 2012 at Samsung Medical Center, the population of interest for this study consisted of those patients who were diagnosed with metastatic metachronous lesions with the malignant tumor confirmed histologically. Patients with direct invasion of tumors such as colorectal cancer or gastric cancer to the pancreas head, bile duct, and duodenum were excluded. Patients who underwent pancreaticoduodenectomy of periampullary cancer (malignant tumors of the pancreatic head, bile duct, duodenum, and ampulla of Vater) were also excluded. We retrospectively analyzed patients' gender, age, type and treatment stage of the primary tumor, interval between the primary tumor surgery and the second pancreaticoduodenectomy, symptoms at the time of the diagnosis of metastasis, complications, survival, and survival period after the pancreaticoduodenectomy from the medical record data of Samsung Medical Center.
Descriptive statistics were used for continuous variables. Mean, median, and standard deviation were assessed. Cumulative survival rate after the detection of metastasis or recurrence of the primary tumor was calculated by Kaplan-Meier curve method. Comparisons between groups used the log rank test for univariate analysis of factors that affected the survival rate. Statistical analyses were performed using SPSS PAWS software (ver. 18). Statistical significance was considered when p-value was less than 0.05.
Of 1,045 patients who underwent pancreaticoduodenectomy between January 2000 and December 2012 at Samsung Medical Center, 12 had metastasis with the primary cancer histologically confirmed. The 12 patients included 4 with renal cell carcinoma, 3 with colon cancer (adenocarcinoma), 2 with advanced gastric cancer, 1 with lung cancer (adenocarcinoma), and 1 with breast cancer (invasive ductal carcinoma). All patients underwent surgical treatment for the primary tumor.
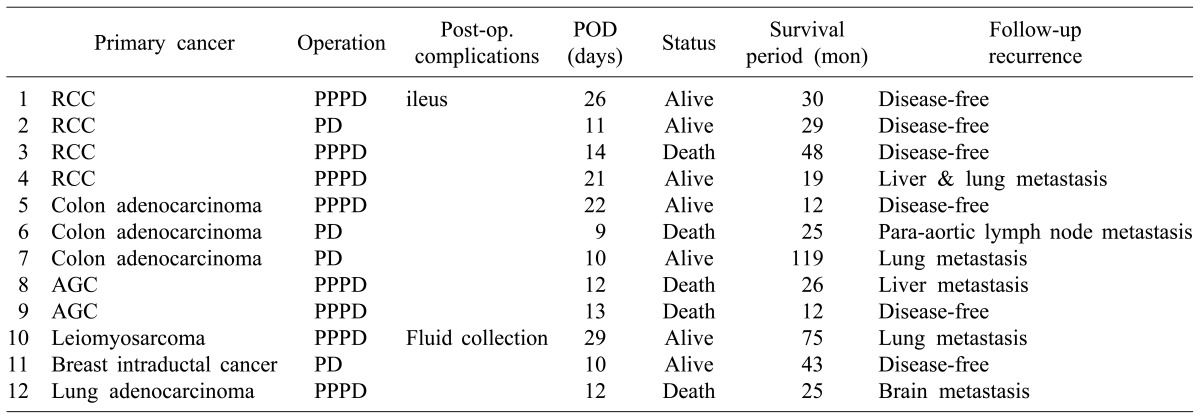
Of the 12 patients who underwent pancreaticoduodenectomy for metastatic cancer, four patients underwent Whipple's operation whereas eight patients underwent pylorus-preserving pancreaticoduodenectomy (PPPD). The average age at the time of surgery was 62.7 years (median 62 [range 50-74]). After surgery for the primary tumor, the average period until the metastasis was found was 67.7 months (median 103 [range 15-176]). Four patients with primary renal cell carcinomas developed metastasis at a period of more than 80 months (Table 2).
Of the 12 patients, 7 had specific symptoms when they were diagnosed with metastasis; the other 5 were diagnosed through regular follow-ups. Of the 7 patients, symptoms included jaundice (n=3), abdominal pain (n=2), and general weakness (n=2). There was no statistically significant relationship between survival time and symptoms. However, when patients had jaundice, their survival time was shorter than patients with abdominal pain or no symptom (29.3 months survival time for jaundice and 59.9 months for asymptomatic or other symptoms; p=0.393).
The stage of primary cancer during the treatment period is summarized in Table 1. In two patients (1 sarcoma, 1 renal cell carcinoma), we were unable to be determine the stage due to missing operative records from other hospitals. Between each period until metastasis after the treatment according to the stage of primary tumor, no difference was observed. The metastasis areas of malignant tumors were: pancreas head position (n=7), common bile duct (n=7), and duodenum (n=2). There was no metastasis to other organs.
The average survival period after the pancreaticoduodenectomy was 38.6 months (median 65.6 [range 12-119]). Until December of 2013, of the 12 patients, seven have survived and five (1 case of renal cell carcinoma, 1 case of colorectal cancer, 2 cases of advanced gastric cancer, 1 case of lung cancer patients) have died. In terms of survival, three patients with renal cell carcinoma had an average survival time of 60 months. For patients with renal cell carcinoma or colorectal cancer, the survival period after the pancreaticoduodenectomy was longer than those with other primary cancers (mean 63.7 vs. 29.6 months; p=0.251) (Table 3).
No mortality due to surgery occurred. In postoperative complications, one patient suffered from ileus and one showed fluid collection in the operative site. They were discharged after improvements with conservative treatment. Severe complications such as pancreatic leakage or pseudoaneurysm were not observed.
Pancreaticoduodenectomy of metastatic cancer excluding periampullary cancer is rare. In the past, this surgery was technically complex and difficult to deliver benefits to patients. Patients usually underwent other treatments such as chemotherapy or radiotherapy when metastatic cancer was found. Moreover, surgeries are frequently difficult because the pancreatic metastasis is often accompanied by metastases to other organs.6
Although the efficacy of pancreaticoduodenectomy for metastatic tumors has not been clearly established, it has been confirmed in several studies that the prognosis could be improved through surgical treatment in patients with primary renal cell carcinoma metastasis to the pancreatic area.7,8 In the case of renal cell carcinoma, recurrence or metastasis to lung, liver, and bone is up to 20-30%, with metastasis to pancreatic sites less common. In one case of renal cell carcinoma of the 12 patients in our study, liver and lung metastases were detected after pancreaticoduodenectomy. Thus, differentiation from the primary tumor and histological identification is necessary before the surgical treatment.
When we analyzed the interval between the primary treatment and pancreaticoduodenectomy, there was no significant relationship between the survival period and the time metastatic cancer was detected. When metastatic cancer was detected more than 5 years after the primary operation, the survival time was 51.5 months. When metastatic cancer was detected less than 5 years after the primary operation, the survival time was 48.0 months (p=0.324). In this study, because the population had only 12 patients, there was no statistically significant difference between many variables, such as survival period and survival rate. However, our results did show that the survival rate after pancreaticoduodenectomy was affected by the type of primary tumor. In the case of renal cell carcinoma and colorectal cancer, the average survival period after pancreaticoduodenectomy was 63.7 months, longer than other primary tumor patients (mean 29.6 months). This is consistent with other studies.9,10 Therefore, when surgical treatment of the primary tumor and metastatic tumor is possible, the prognosis of patients can be improved.
Of patients who had symptoms at the time of metastatic cancer detection, five with jaundice symptom had worse short-term survival than other patients with abdominal pain or no symptom (29.3 vs. 59.9 months; p=0.393). Abdominal pain or general weakness were non-specific symptoms, therefore, relationship between these symptoms and metastasis of the primary tumor was difficult to determine. However, patients with jaundice could have developed obstructive jaundice due to the metastatic tumor. We cannot predict metastasis location or symptoms. However, regular follow-up examinations are important through on-going check-ups. Surgical and/or medical treatments could be used before the symptoms of jaundice affect the prognosis of patients. We assessed the progress of patients with stage M1 (TNM staging) of the primary tumor. In the progress of patients after pancreaticoduodenectomy, the average survival rates in cases of a single pancreatic metastatic tumor were improved in most cases compared to that of a stage M1 primary tumor.11,12
This study has some limitations. First, this was a retrospective study conducted at one institution with a target patient group of 12 patients. Thus, it was simply impossible to analyze the progress of patients with recurrence or metastasis of the primary tumor after pancreaticoduodenectomy effectively. It is possible that it contained a selection bias when choosing 12 patients of study group because they had operability for palliative surgery. Second, according to the type of the primary tumor after excluding renal cell carcinoma and colon cancer, each patient group consisted of one person. Therefore, recurrence, metastasis, progress, and outcomes could not be analyzed meaningfully. Third, in a retrospective study, when metastasis and recurrence of a primary tumor occurred, it was difficult to make a direct comparison on prognosis according to surgical treatment, radiation therapy, or chemotherapy because a single metastasis to the pancreas head area was rare. Most patients received medical therapy for accompanying metastases to other organs.
Recently, in cases that require surgical treatment for malignant tumors of the pancreas and biliary tract, the risk of pancreaticoduodenectomy is reported to be lower with sufficient stability to promote survival and prognosis.1,2 In this study, no serious complication occurred in any of the 12 patients. Only two cases complications such as effusion at the operative site and ileus occurred. However, these complications were improved and patients were discharged in stable state within 30 days after the surgery.
In summary, pancreaticoduodenectomy is not commonly performed in patients with metastatic cancer. Chemotherapy and radiation therapy were commonly used for many patients. Studies on the prognosis of surgical treatment have not been reported sufficiently. There is still controversy regarding the prognosis and the effectiveness of surgical treatment. If the indications for surgical treatment of patients with metastatic cancer and the prognosis are evaluated, pancreaticoduodenectomy does appear to have sufficient survival benefit. Therefore, it should be considered as one of the main treatments.
References
1. Chen SC, Shyr YM, Wang SE. Longterm survival after pancreaticoduodenectomy for periampullary adenocarcinomas. HPB (Oxford). 2013; 15:951–957. PMID: 23472708.

2. Huang JJ, Yeo CJ, Sohn TA, Lillemoe KD, Sauter PK, Coleman J, et al. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg. 2000; 231:890–898. PMID: 10816633.

3. Z'graggen K, Fernández-del Castillo C, Rattner DW, Sigala H, Warshaw AL. Metastases to the pancreas and their surgical extirpation. Arch Surg. 1998; 133:413–417. PMID: 9565122.
4. Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001; 51:686–690. PMID: 11696171.

5. Klein KA, Stephens DH, Welch TJ. CT characteristics of metastatic disease of the pancreas. Radiographics. 1998; 18:369–378. PMID: 9536484.

6. Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004; 444:527–535. PMID: 15057558.

7. Law CH, Wei AC, Hanna SS, Al-Zahrani M, Taylor BR, Greig PD, et al. Pancreatic resection for metastatic renal cell carcinoma: presentation, treatment, and outcome. Ann Surg Oncol. 2003; 10:922–926. PMID: 14527912.

8. Kassabian A, Stein J, Jabbour N, Parsa K, Skinner D, Parekh D, et al. Renal cell carcinoma metastatic to the pancreas: a single-institution series and review of the literature. Urology. 2000; 56:211–215. PMID: 10925080.

9. Crippa S, Angelini C, Mussi C, Bonardi C, Romano F, Sartori P, et al. Surgical treatment of metastatic tumors to the pancreas: a single center experience and review of the literature. World J Surg. 2006; 30:1536–1542. PMID: 16847716.

10. Hiotis SP, Klimstra DS, Conlon KC, Brennan MF. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002; 9:675–679. PMID: 12167582.

11. Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM, et al. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol. 2008; 15:3199–3206. PMID: 18784960.

12. Sperti C, Pasquali C, Liessi G, Pinciroli L, Decet G, Pedrazzoli S. Pancreatic resection for metastatic tumors to the pancreas. J Surg Oncol. 2003; 83:161–166. PMID: 12827684.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download