Abstract
Backgrounds/Aims
Gallbladder carcinoma is usually associated with an unfavorable prognosis, and the clinical outcome has not improved much. This study was conducted to evaluate outcomes with gallbladder carcinoma according to the type of surgery performed, and the prognostic factors for survival.
Methods
One hundred and six patients with gallbladder carcinoma, who underwent surgery for the purpose of curative resection between January 1999 and June 2012 were reviewed retrospectively.
Results
Out of 106 patients, curative resection was achieved in 75 (70.8%). The cumulative 1-, 2- and 5-year survival rates of the gallbladder carcinoma patients were 93.4%, 80.9% and 63.0%, respectively. Radical resections, including extended cholecystectomy, were more beneficial for long term survival of patients. The 5-year survival rate in patients who underwent curative resection (56.9%) was significantly higher than in those who underwent palliative resection (0%, p=0.000). Multivariate analysis revealed that curative resection, preoperative CA19-9, T-stage, N-stage and differentiation of histology were independently significant prognostic factors.
Go to : 
Gallbladder carcinoma is difficult to diagnose early without suspicion in the initial stage of occurrence. When a diagnosis is made, radical surgery often cannot be performed because of frequent invasions into important circumferential structures such as the hepatic artery or the portal vein.1 It is known that the prognosis for advanced gallbladder cancer is poor despite aggressive treatment.2,3 The 5-year survival rate was reported to be very poor in the past, but according to the result of recent studies, the 5-year survival rate of overall gallbladder carcinoma has improved significantly.3,4,5,6 This improvement is likely affected by not only increased early diagnosis due to the development of image technology, but also the introduction of extensive active resections such as extended cholecystectomy including lymphadenectomy, massive hepatectomy, and pancreaticoduodenectomy in addition to simple cholecystectomy.7,8,9
However, the effects of these methods on long-term prognosis are not consistent, and there is no completely agreed upon opinion on the application of proper surgical methods according to progression of the tumor.4,10,11 Based on the recent report that extending the surgery range is adequate in cases where residual tumor cannot be left and active surgery showed better prognosis,7,8,9,12 extended cholecystectomy, including regional lymph node dissection, and hepatectomy when hepatic invasion is suspected are recognized as the radical treatment options for standard gallbladder carcinoma.
We analyzed various surgical methods and survival rates depending on progression stages for gallbladder carcinoma patients who received surgery for the purpose of curative resection. The prognostic factors affecting survival of the patients were analyzed from the medical report after the surgery.
Go to : 
The medical records of 106 patients who received surgery for the purpose of curative resection from January 1999 to June 2012 at the Department of Surgery, Gachon University Gil Medical Center and were histologically diagnosed with adenocarcinoma were analyzed retrospectively. Out of 121 patients who received the surgery, 6 patients who died after the surgery, 6 who could not receive curative resection because metastasis was found within the abdominal cavity, and 3 who were diagnosed with small cell carcinoma and squamous cell carcinoma in the biopsy performed after the surgery were excluded from the prognostic factor analysis. The gender, age, clinical findings, laboratory findings before and after the surgery, surgical methods, and pathologic findings after the surgery were investigated from the medical record.
For the stages of gallbladder carcinoma, the classification of AJCC (American Joint Committee on Cancer, 7th edition) was used. Radical resection was defined as surgery in which complete resection of the tumor is noted in operative fields and no residual cancer cell is found in the resection margin after pathologic examination.
SPSS 17.0 Window Package was used for statistical analysis, and the survival rate and curve were calculated using the Kaplan-Meier method, then compared through the Log-rank method. After conducting univariate analysis for prognostic factors, multivariate analysis was conducted for significant factors using Cox's regression hazard model. The interpretation of significance was set as p less than 0.05.
Go to : 
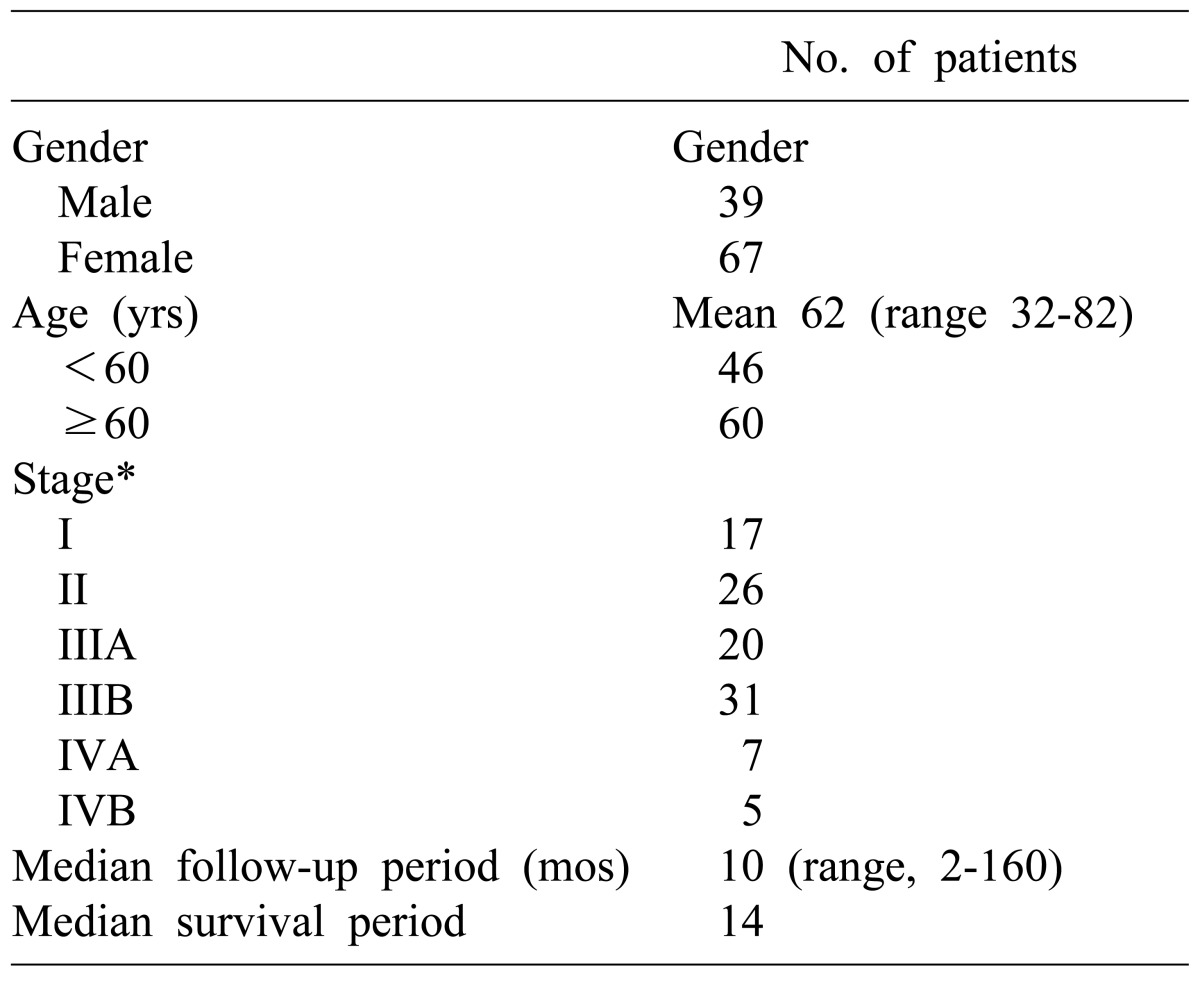
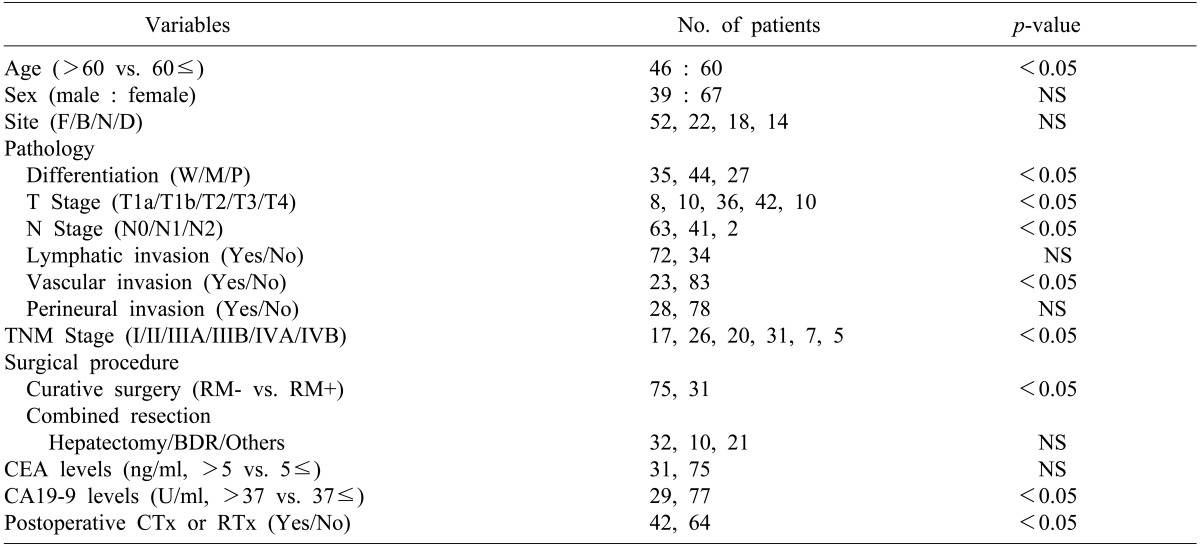
The mean age of the patients was 62 years (range, 32-82 years). The number of patients aged between 60 and 70 was 60 (56.6%), 46 were younger than 60 (43.4%), and patients over 70 were the least common (27.4%). There were 39 males (36.8%), and 67 females (63.2%).
For the preoperative clinical symptoms, abdominal pain was the most common and was found in 66 patients (62.3%), followed by gallbladder mass found accidentally in 19 patients during health examination (17.9%), and jaundice in 10 patients (9.4%). The number of patients who were diagnosed with gallbladder carcinoma in specimen pathology after being diagnosed with cholecystitis and receiving cholecystectomy was 14 (13.2%).
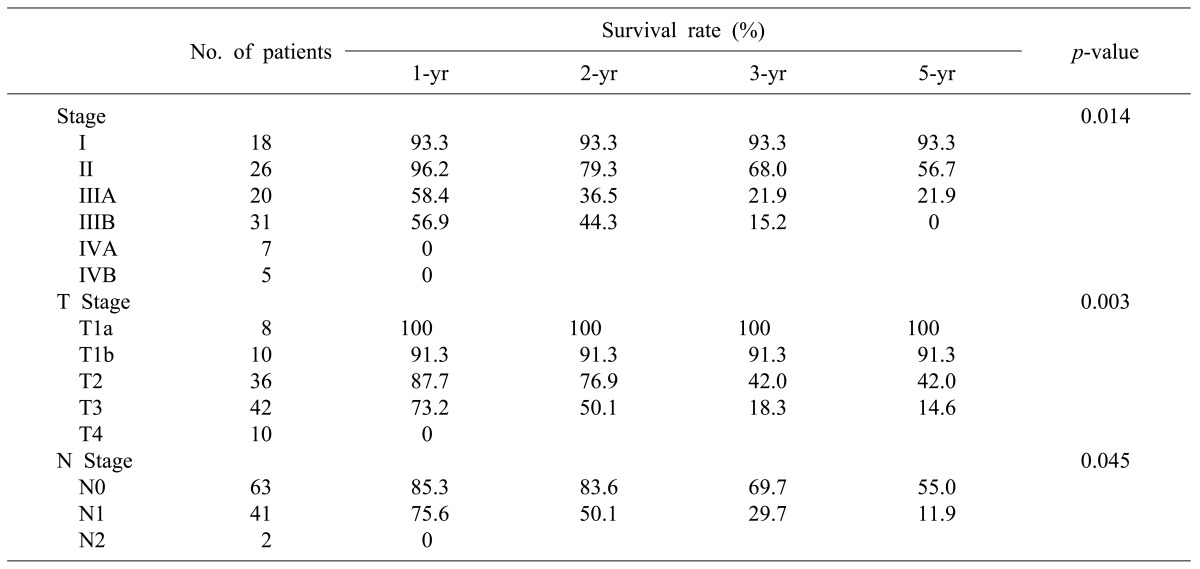
Distribution of patients by stage according to the TNM classification of AJCC was as follows: 17 patients were at stage I, 26 were stage II, 20 were stage IIIA, 31 were stage IIIB, 7 were stage IVA, and 5 were Stage IVB, respectively. The number of patients who had progressed beyond stage III were 63 (59.4%), which was more than half (Table 1).
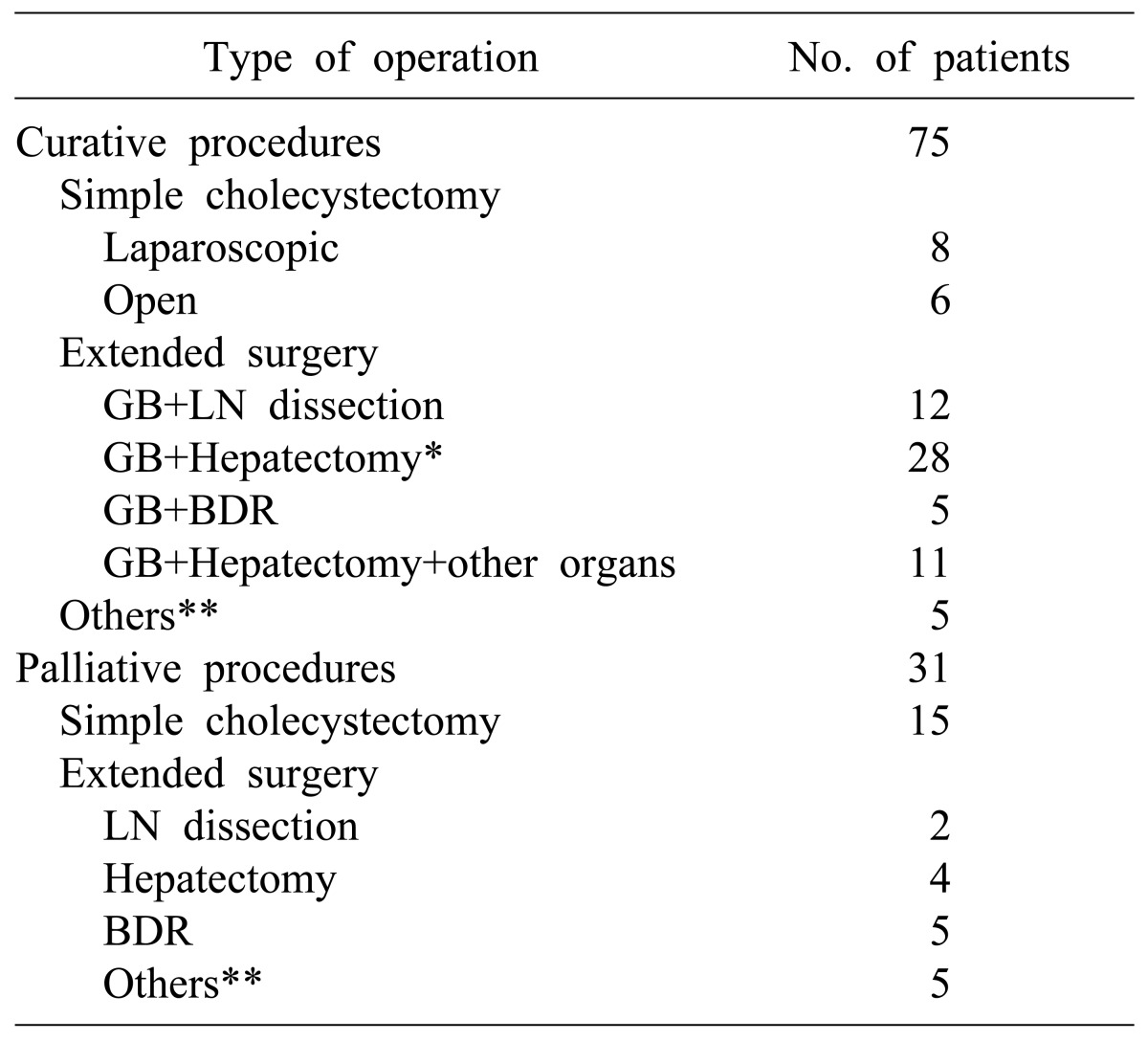
Out of 106 patients, curative resection was conducted in 75 patients (70.8%), and tumor remaining in the resection margin was found in 31 patients (29.2%) (Table 2). Among the patients who received curative resections, all 14 patients in stage I received only cholecystectomy. Surgeries for the 61 patients who received extended cholecystectomy included regional lymphadenectomy in 12 patients, liver wedge resection or hepatectomy including S4b+S5 in 28 patients, bile duct resection and choledochojejunostomy in 5 patients, and major hepatectomy and other organ resection in 11 patients. In addition, pancreaticoduodenectomy due to invasion of the duodenum was conducted in 3 patients, and 2 patients received colon resection. Among 31 patients who did not receive radical resection and were positive in the resection margin according to histological examination, simple cholecystectomy only was conducted in 15 patients. Extended surgery was suggested for 4 of them because they were diagnosed with more than T2 stage, but consent could not be obtained from the patients and guardians. Therefore, the patients were classified as no radical resection. Surgeries for the 16 patients who received extended surgery included cholecystectomy and local lymphadenectomy in 2 patients, hepatectomy and local lymphadenectomy in 4 patients, and bile duct resection and choledochojejunostomy in 5 patients. The remaining 5 patients who received colon resection or pancreatic resection were classified as no radical resection because remnant tumor was found in the resection margin.
The main complications after surgery included infection in the surgery site in 22 patients (20.8%), pneumonia and pulmonary edema in 8 patients (7.5%), and bile leakage in 7 patients (6.6%). Among the 121 patients who received surgery for the purpose of curative resection, 6 died after the surgery (5%). Of these patients, radical resection was conducted in 2 patients, and the remaining 4 received palliative surgery. In the pathological stages, 1 patient in stage II died from postoperative bleeding, and 1 patient in stage IIIA and another in stage IIIB died from postoperative pneumonia complications. One patient in stage IVA and 2 in stage IVB died from multiple organ dysfunction after receiving palliative surgery.
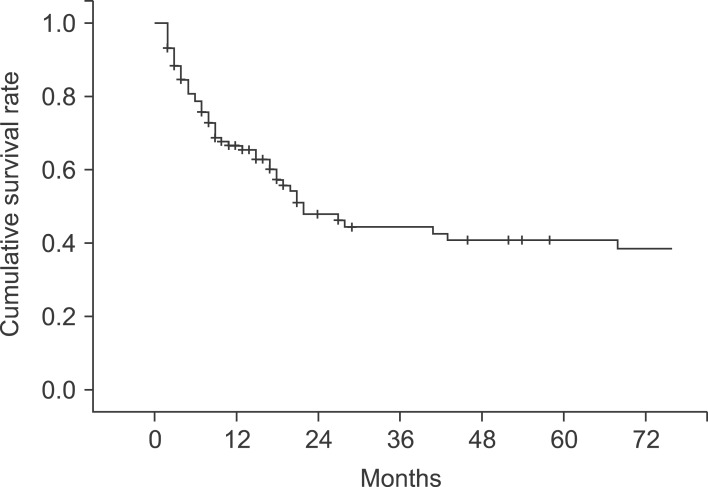
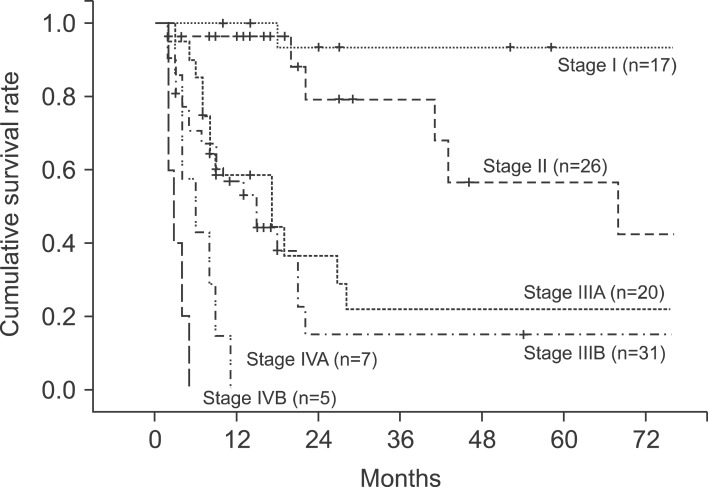
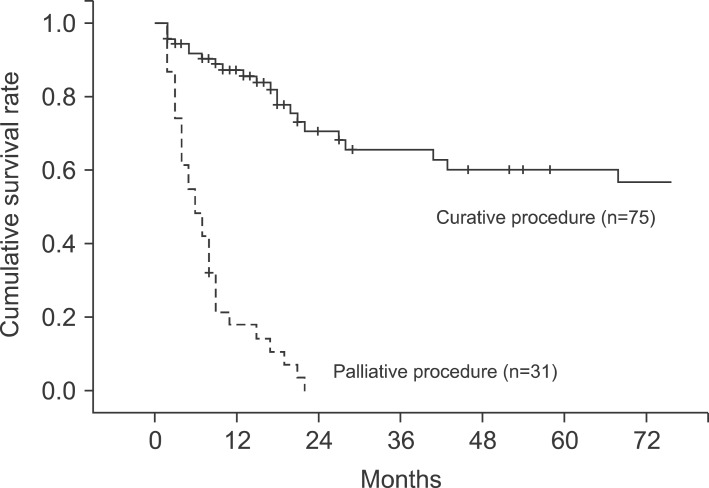
The median follow-up period for the patients was 10 months (range, 2-160 months). The 1-, 2-, 3-, and 5-year cumulative survival rates of 106 patients who received surgery and could be followed-up on were 93.4%, 80.9%, 73.0%, and 63.0%, respectively. The cumulative survival rate of all patients was 38.1% (Fig. 1) and the median survival period was 14 months. For the 5-year survival rate by cancer stage, stages I, II, and IIA were 93.3%, 56.7%, and 21.9%, respectively, and no long-term survivor was found in the patient group above stage IIIB (Table 3, Fig. 2). The 1-, 2- and 3-year survival rates of the patient group without residual tumor after the surgery (curative procedure) were 90.5%, 82.8%, and 56.9%, while the survival rates of patients who had residual tumor and palliative procedure were 61.3%, 32.3%, and 0%, indicating that the patient group that received curative surgery had significantly higher survival (p=0.0003) (Fig. 3).
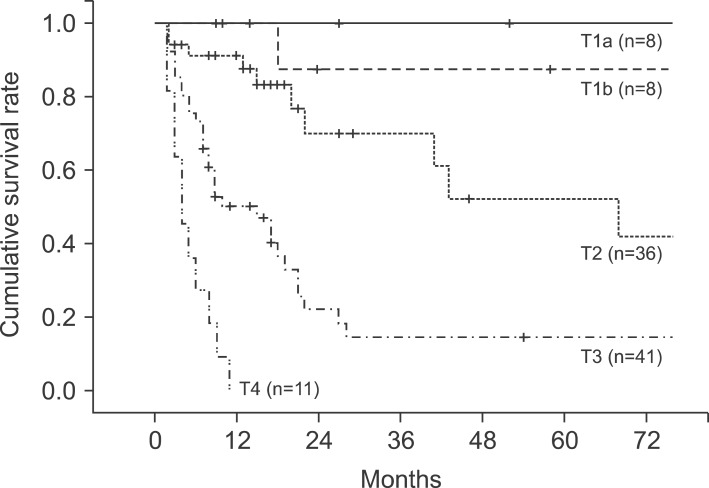
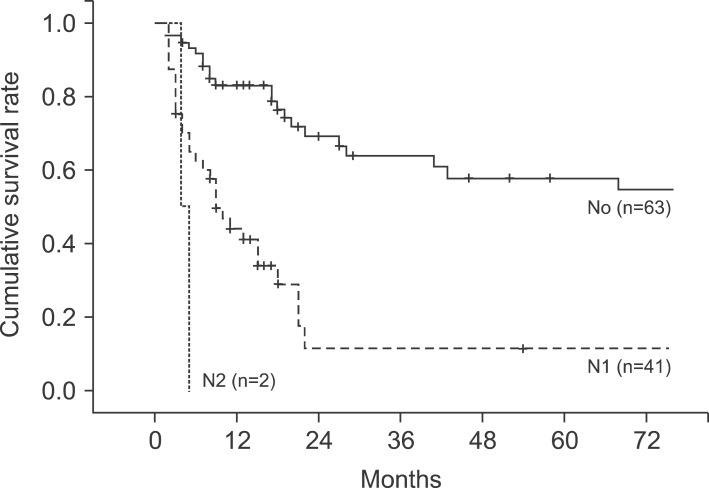
In the pathological findings, 44 patients showed moderate differentiation (41.5%), which was the largest proportion. Patients with poor differentiation had significantly low survival rates. Regarding the T stages, which represent the invasion of the cancer to the gallbladder wall, T3 stage was found in 41 patients (38.7%). This stage was most common, followed by the T2 stage with 36 patients (34%), T1b stage with 10 patients, and the T1a stage with 8 patients. A total of 43 patients (40.6%) had metastasis in their lymph nodes, with 1 patient in whom the tumor invaded muscle in the T1b stage. Out of 36 patients in the T2 stage, 8 (22.2%) had lymph node metastasis (Table 4). The 5-year survival rates in T1a, T1b, T2, T3, and T4 stages were 100%, 91.30%, 42.0%, 14.6%, and 0%, respectively, (p=0.0032), showing statistically significant differences in survival (Fig. 4). The 1-, 3-, and 5-year survival rates in the patient group without lymph node metastasis were 85.3%, 69.7%, and 55.0%, respectively, while the 1-, 3-, and 5-year survival rates of the patient group with N1 metastasis were 75.6%, 29.7%, and 11.9%, indicating that the survival rates of the patient group with lymph node metastasis were significantly low (p=0.045) (Fig. 5). Both patients with N2 lymph node metastasis died within 1 year.
The survival rates of 77 patients in stages II, IIIA, and IIIB were compared. The 18 patients in stage I were excluded from the comparison because they were in the early cancer stage. Another 12 patients in stages IVA and IVB were also excluded because long-term survival at these stages is difficult. The 1-, 2-, and 3-year survival rates of the patients in stage II were 96.2%, 79.3%, and 68.0%, those of the patients in stage IIIA were 58.4%, 36.5%, and 21.9%, and those of the patients in stage IIIB were 56.9%, 44.3%, and 15.2%, respectively (Table 3). The patient groups in stages II and III showed a significant difference in survival rates, but there was no statistically significant difference in survival rate between the patient groups in stages IIIA and IIIB (p=0.465). Of the 77 patients with advanced gallbladder carcinoma in stages II and III, 58 (75.3%) received curative resection without residual tumor after the surgery and the other 19 had residual tumor, showing a significant difference in survival rate according to the status of the resection margin (p=0.00032).
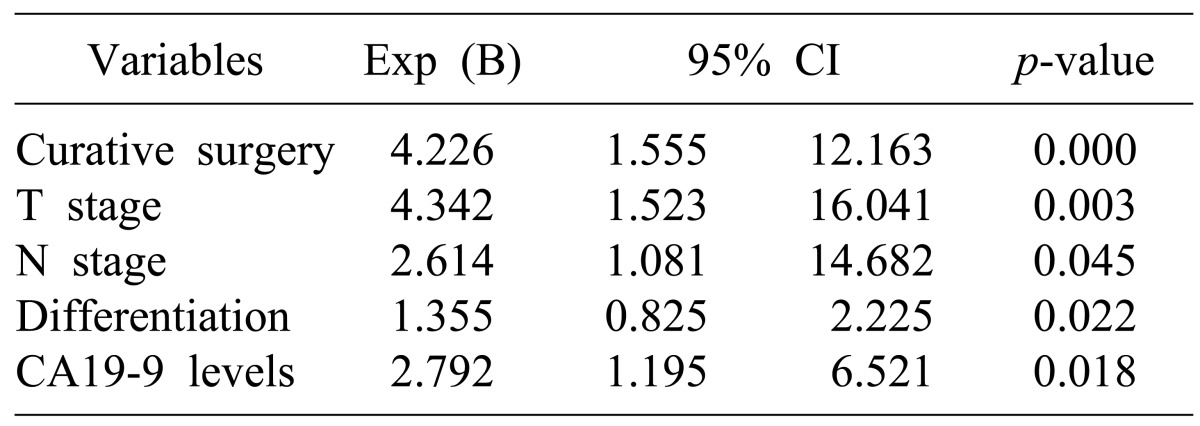
The factors affecting survival rates were analyzed in the 106 patients who were able to receive follow-up after the surgery. In the univariate analysis, if the age of the patient was 60 or more, the pathological differentiation of tumor, depth of tumor invasion (T stage), lymph node metastasis (N stage), arteriole invasion, residual tumor in resection margin, preoperative CA19-9 level, additional chemotherapy after surgery, and radiation treatment were the factors with significant effects. Multivariate analysis using the Cox proportional hazard model showed that curative resection, depth of tumor invasion (T stage), lymph node metastasis (N stage), poor differentiation of tumor, and preoperative CA19-9 level of 37U/ml or higher were the independent factors that affected survival rates (Tables 4, 5).
Go to : 
The depth of tumor invasion, lymph node metastasis, histologic type of cancer cells, and TNM stages are mentioned as the prognostic factors affecting clinical prognosis of patients with gallbladder carcinoma. Like other digestive system cancers, curative resection of the tumor is the most critical factor.1,5,6 Most studies report that 5-year survival rate was poor because early diagnosis is difficult due to the non-specificity of the clinical symptoms and the disease frequently occurs in the elderly population. Histologically, the radical resection rate is low due to a lot of organ or liver hilum invasion starting from a relatively early stage because of the anatomical structure of the gallbladder, which has no submucosal layer.3,8,11 Also, because of abundant lymph nodes adjacent to the gallbladder, it is easily metastasized to the lymph nodes around the bile duct, perihilar and pancreaticoduodenal areas. With frequent liver metastasis through the venous pattern in the liver, resection can be difficult.12,13,14 Therefore, various surgical methods are attempted depending on the progress of the lesion. For stage T1a, in which the tumor is limited to the mucosa layer, it is generally accepted that simple cholecystectomy may be enough. However, if the tumor has progressed further, standard surgical methods are not consistent.
If advanced gallbladder carcinoma is diagnosed, resection of the liver adjacent to the gallbladder bed along with cholecystectomy and lymphadenectomy around the bile duct, portal vein, and hepatic artery are standard surgical methods. However, reports vary on whether the extension of the resection range promotes prognosis. Cubertafond et al.2 investigated 724 gallbladder carcinoma patients and diagnosed 85% of the patients as lesions T3 and T4, reporting that their 5-year survival rate was only 5% because curative resection rate was only 23%. Also, they stated that because the survival rates of patients with advanced gallbladder carcinoma could not be improved by extended surgery including massive hepatic resections, extensive resection greater than the extended cholecystectomy applied in the existing literature was useless for lesions at T3 stage or higher. In the past, such extended surgery had to be carefully decided because it did not help prognosis as much as it increased operative mortality rate and complications.2,15,16
Recently, however, early diagnosis has increased due to the development of diagnostic technology, and major hepatic resection, combined bile duct resection, and pancreaticoduodenectomy are being conducted with relative safely. The number of studies showing that active resection contributes to the improvement of prognosis are increasing.7,8,9,12,17 Recently, the importance of curative resection without leaving residual tumor regardless of operation range is more emphasized. There are some reports that with liver metastasis of gallbladder carcinoma or pancreas invasion considered impossible for resection, a 5-year survival rate around 15% can be expected if curative resection is possible through pancreaticoduodenectomy along with massive liver resection.8,9 This outcome is much better than the survival rates of the groups that received palliative resection.
There are a number of reports that curative resection and survival rates in the patients that received extended surgery have dramatically improved in Korea.18,19,20 We found that among 75 patients who received radical resection, 49 received extended surgery including hepatectomy, bile duct resection, or adjacent organ resection, encompassing 65% of all patients. When comparing only 77 patients in stages II and III, classified according to the AJCC 7th edition, to determine the significance of extended surgery for advanced gallbladder carcinoma patients, 59 patients received curative resection, and 19 patients had positive residual tumor. Among them, the 1-, 2-, and 3-year cumulative survival rates of the patients without residual tumor were 61.9%, 33.7%, and 16.9%, and those with positive residual tumor were 42.8%, 7.1%, and 0%, respectively, showing statistically significant difference. These results suggest that long-term survival beyond 3 years cannot be expected in patients in stages II and III with positive residual tumor, which is similar to the results obtained by other reports, indicating that radical resection may be the most important prognostic factor. However, when the patients in stage III were divided were divided into stages IIIA and IIIB depending on lymph node metastasis, according to the AJCC 7th edition, the current study confirmed no significant difference in survival rates between the 2 groups. Further study is required on this topic with many more patient groups in the future.
There were 12 patients diagnosed with stage IV in pathologic examination after surgery. Although there were multiple invasions to peripheral organs, portal vein and hepatic artery invasions, intraperitoneal metastasis were not found. Ten stage T4 patients had invasions to adjacent organs and another 2 patients had multiple lymph node metastasis. The median survival period of stage IV patients who received extensive resection was 4 months (2-11 months), and there was no long-term survivor who survived for more than 1 year. Therefore, extensive surgery should be applied carefully if adjacent organ or main vessel invasions are severe or if there is extensive lymph node metastasis in the perioperative examination.
The factors related to prognosis, similar to other reports, include curative resection, preoperative CA19-9 level, T stage, N stage, and differentiation of tumor tissue, which have independent effects. It may be that lymph node metastasis, which acts as a prognostic factor in the overall patient group, shows no effect in stage III patients because the number of patients was too small to distinguish lymph node metastasis in individual stage patients.
The gross finding, in addition to TNM classification, is known as an important prognostic factor. If the gross finding of gallbladder carcinoma is infiltrative after curative resection, it can be expected that tissue differentiation is bad, and the carcinoma is likely to be at an advanced stage.19 Although the prognosis may differ depending on the differentiation of the cancer cell, there was a recent report that cell differentiation itself does not affect prognosis for stages over T3 with invasion exceeding the gallbladder wall.21 In the pathologic findings investigated in our hospital, the gross findings were not described in detail and its correlation could not be found. This seems to require further study in the future.
Currently, the extent of surgical resection for gallbladder carcinoma is determined by T stage. For T1 lesions, it is known that the 5-year survival rate is higher than 90% with only a simple cholecystectomy, and it is accepted that conducting only laparoscopic or open cholecystectomy is radical surgery for T1a lesions. In contrast, T1b lesions which invaded the muscle layer had some controversies. Because lymph node metastasis around the hepatoduodenal ligament is found in 3-20% patients with T1b lesions, the need for lymphadenectomy is greater in order to obtain curative resection and accurately identify the stage.22,23 However, there have been important reports that there is no difference in survival rate between patients who received simple cholecystectomy and those that received extended cholecystectomy including lymphadenectomy, even when lymph nodes were found in the T1b lesion.24,25 In our study, local lymph node metastasis was diagnosed in 1 patient (14.3%) out of 7 who were in stage T1b and received lymphadenectomy. The 3 patients who were diagnosed with stage T1b after laparoscopic cholecystectomy did not receive additional operation, and their progresses are being observed. All of them survived for a long time. Because it is difficult to accurately determine T stage in preoperative diagnosis, lymphadenectomy is conducted along with open cholecystectomy. There are a number of controversies on additional surgery when gallbladder carcinoma is diagnosed after simple cholecystectomy. If gallbladder carcinoma is suspected when conducting laparoscopic cholecystectomy, open cholecystectomy is recommended, except in cases with extensive metastasis. If it is decided that obtaining a safe margin through only laparoscopy is difficult or that hepatic resection is needed for curative resection by evaluating the abdominal cavity, liver, and lymph node, it is preferable to cease manipulation of the gallbladder to be resected, finish the surgery first and attempt safe reoperation in the future.26,27 In addition, Suzuki et al.28 recommended against performing open surgery immediately, but to finish the surgery first, determine the T stage through permanent pathologic examination, and conduct radical treatment according to the stage. This recommendation stands even if cancer cells are found in frozen section examination as findings suspicious of malignant tumor in the gallbladder sample collected during laparoscopic cholecystectomy for benign gallbladder disease.
When gallbladder carcinoma above T2 stage is diagnosed in pathologic examination after laparoscopic cholecystectomy, radical surgery is needed.29,30 Chijiiwa et al.31 reported that the group of stage T2 patients that received extended cholecystectomy, including hepatic resection, showed more significant improvement in 5-year survival than the patient group that received simple cholecystectomy (59% versus 17%). If findings suspected of gallbladder carcinoma are observed when conducting laparoscopic cholecystectomy, it is most important to avoid perforation in the gallbladder during the resection. Gallbladder perforation is known to be related to recurrence around the site of laparoscope trocar or disseminated metastasis in the abdominal cavity.26,32 The number of patients who received only simple cholecystectomy through laparoscope or laparotomy and were diagnosed with more than stage T2 in pathologic examination was 12. They received extended cholecystectomy again, and all of them were eligible for radical resection.
To increase the rate of curative surgery for gallbladder carcinoma, it is enormously important to determine the preoperative and postoperative tumor stage and apply the proper surgical method. Also, as the authors have experienced, the application of extensive surgery should be determined through careful consideration of the risk of surgery and the possibility of survival improvement in advanced gallbladder carcinomas such as stages IVA and IVB.
Go to : 
References
1. Park JI, Kim JS, Kim KH, Kim KW, Kim KH, Choi CS, et al. Analysis of prognostic factors affecting survival in patients with gallbladder carcinoma. Korean J Hepatobiliary Pancreat Surg. 2010; 14:173–183.
2. Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994; 219:275–280. PMID: 8147608.

3. Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, et al. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007; 14:833–840. PMID: 17103074.

4. Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997; 79:892–899. PMID: 9041150.

5. Noshiro H, Chijiiwa K, Yamaguchi K, Shimizu S, Sugitani A, Tanaka M. Factors affecting surgical outcome for gallbladder carcinoma. Hepatogastroenterology. 2003; 50:939–944. PMID: 12845954.
6. Choi SB, Han HJ, Kim CY, Kim WB, Song TJ, Suh SO, et al. Fourteen year surgical experience of gallbladder cancer: validity of curative resection affecting survival. Hepatogastroenterology. 2012; 59:36–41. PMID: 22251521.
7. Muratore A, Polastri R, Capussotti L. Radical surgery for gallbladder cancer: current options. Eur J Surg Oncol. 2000; 26:438–443. PMID: 11016462.

8. Shimizu H, Kimura F, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, et al. Aggressive surgical approach for stage IV gallbladder carcinoma based on Japanese Society of Biliary Surgery classification. J Hepatobiliary Pancreat Surg. 2007; 14:358–365. PMID: 17653633.

9. Chijiiwa K, Kai M, Nagano M, Hiyoshi M, Ohuchida J, Kondo K. Outcome of radical surgery for stage IV gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2007; 14:345–350. PMID: 17653631.

10. Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998; 175:118–122. PMID: 9515527.
11. Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg. 2008; 207:371–382. PMID: 18722943.

12. Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Extensive surgery for carcinoma of the gallbladder. Br J Surg. 2002; 89:179–184. PMID: 11856130.

13. Donohue JH. Present status of the diagnosis and treatment of gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2001; 8:530–534. PMID: 11956904.
14. Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am. 2007; 16:221–232. PMID: 17336245.

15. Chao TC, Wang CS, Jeng LB, Jan YY, Chen MF. Primary carcinoma of the gallbladder in Taiwan. J Surg Oncol. 1996; 61:49–55. PMID: 8544460.

16. Yamamoto M, Onoyama H, Ajiki T, Yamada I, Fujita T, Saitoh Y. Surgical results of operations for carcinoma of the gallbladder. Hepatogastroenterology. 1999; 46:1552–1556. PMID: 10430293.
17. Kondo S, Nimura Y, Kamiya J, Nagino M, Kanai M, Uesaka K, et al. Factors influencing postoperative hospital mortality and long-term survival after radical resection for stage IV gallbladder carcinoma. World J Surg. 2003; 27:272–277. PMID: 12607050.

18. Lee KY, Ahn SI, Choi YM, Lee KY, Cho YU, Choi SK, et al. Analysis of prognostic factors after surgery for gallbladder cancer. J Korean Surg Soc. 2005; 69:476–481.
19. Park JS, Yoon DS, Kim KS, Choi JS, Lee WJ, Chi HS, et al. Analysis of prognostic factors after curative resection for gallbladder carcinoma. Korean J Gastroenterol. 2006; 48:32–36. PMID: 16861879.
20. Yoo KE, Jo SH, Heo JS, Choi SH, Kim YI, Moon HJ, et al. Surgical outcome and prognostic factors of primary gallbladder carcinoma. J Korean Surg Soc. 2004; 67:384–389.
21. Roa I, Ibacache G, Muñoz S, de Aretxabala X. Gallbladder cancer in Chile: pathologic characteristics of survival and prognostic factors: analysis of 1,366 cases. Am J Clin Pathol. 2014; 141:675–682. PMID: 24713738.
22. You DD, Lee HG, Paik KY, Heo JS, Choi SH, Choi DW. What is an adequate extent of resection for T1 gallbladder cancers? Ann Surg. 2008; 247:835–838. PMID: 18438121.

23. Fetzner UK, Hölscher AH, Stippel DL. Regional lymphadenectomy strongly recommended in T1b gallbladder cancer. World J Gastroenterol. 2011; 17:4347–4348. PMID: 22090793.

24. Lee SE, Jang JY, Kim SW. The surgical strategy for treating T1 gallbladder carcinoma. Korean J Hepatobiliary Pancreat Surg. 2009; 13:69–75.
25. Lee SE, Jang JY, Kim SW, Han HS, Kim HJ, Yun SS, et al. Korean Pancreas Surgery Club. Surgical strategy for T1 gallbladder cancer: a nationwide multicenter survey in South Korea. Ann Surg Oncol. 2014; 21:3654–3660. PMID: 24743905.

26. Box JC, Edge SB. Laparoscopic cholecystectomy and unsuspected gallbladder carcinoma. Semin Surg Oncol. 1999; 16:327–331. PMID: 10332779.

27. Romano F, Franciosi C, Caprotti R, De Fina S, Porta G, Visintini G, et al. Laparoscopic cholecystectomy and unsuspected gallbladder cancer. Eur J Surg Oncol. 2001; 27:225–228. PMID: 11373097.
28. Suzuki K, Kimura T, Ogawa H. Long-term prognosis of gallbladder cancer diagnosed after laparoscopic cholecystectomy. Surg Endosc. 2000; 14:712–716. PMID: 10954815.

29. Wakai T, Shirai Y, Hatakeyama K. Radical second resection provides survival benefit for patients with T2 gallbladder carcinoma first discovered after laparoscopic cholecystectomy. World J Surg. 2002; 26:867–871. PMID: 11960212.

30. Park YJ, Hwang S, Kim KH, Lee YJ, Ahn CS, Moon DB, et al. Prognosis of patients with pT1b/T2 gallbladder carcinoma who have undergone laparoscopic cholecystectomy as an initial operation. Korean J Hepatobiliary Pancreat Surg. 2013; 17:113–117.

31. Chijiiwa K, Nakano K, Ueda J, Noshiro H, Nagai E, Yamaguchi K, et al. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg. 2001; 192:600–607. PMID: 11333097.

32. Shirai Y, Ohtani T, Hatakeyama K. Laparoscopic cholecystectomy may disseminate gallbladder carcinoma. Hepatogastroenterology. 1998; 45:81–82. PMID: 9496492.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download