Abstract
Acute acalculous cholecystitis (AAC) is defined as acute inflammation of the gallbladder in the absence of gallstones. AAC occurs in patients after major surgery and in the presence of serious co-morbidities such as severe trauma, burns, sepsis, prolonged intravenous hyperalimentation and hemodynamic instability. AAC is rare in patients with none of the established risk factors. We present a case of a 38-year-old woman who developed AAC after laparoscopic appendectomy.
Acute acalculous cholecystitis (AAC) is defined as acute inflammation of the gallbladder in the absence of gallstones and has a multifactorial pathogenesis.1 AAC occurs in about 10% of all cases of acute cholecystitis. AAC has numerous causes that produce bile stasis and ischemia leading to inflammation and infection of the gallbladder. AAC tends to have a more fulminant course, is frequently associated with gangrene, perforation and empyema, and has high morbidity and mortality.2 AAC has traditionally been recognized to occur in patients with serious co-morbid illnesses especially after a major operation, severe trauma, burns, systemic sepsis and prolonged intravenous hyperalimentation.3 However, there has recently been an increasing number of reports in the literature of the occurrence of AAC in patients with none of the established risk factors.4-6 AAC arising as a complication of laparoscopic appendectomy has been reported in just 1 case and it was treated conservatively.7 Herein, we present the case of a 38-year-old woman who developed AAC after laparoscopic appendectomy.
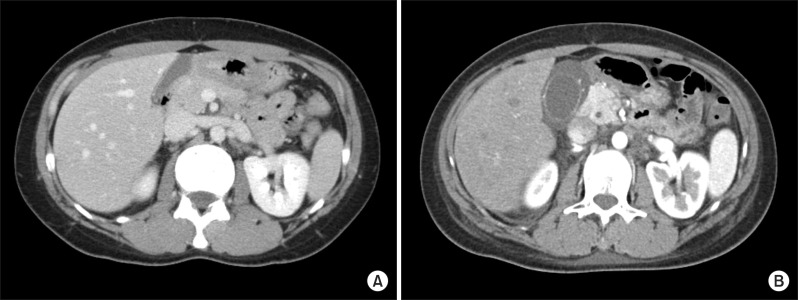
A 38-year-old woman who was previously in good health came to our emergency room 3 days after she received laparoscopic appendectomy at a local clinic. She had acute abdominal pain which initially occurred in the epigastric and umbilical area and then migrated to the right lower quadrant after 10 hours and was associated with nausea and anorexia. Acute appendicitis had been diagnosed by abdominal computed tomography (CT) and operative findings at another hospital. Her abdominal pain and associated symptoms improved after surgery. However, on the second postoperative day, she complained of abdominal pain. Her background history was unremarkable except for the laparoscopic appendectomy. She was on no regular medication, did not smoke or drink alcohol. On physical examination, she had normal blood pressure and pulse. Body temperature was 37.8℃. She exhibited tenderness and guarding in the epigastric region and right upper quadrant. Results of the laboratory studies were unremarkable, except for elevated C-reactive protein (CRP) which was 8.04 mg/dl (normal range less than 0.5 mg/dL). Her body mass index (BMI) was 21.7 (height: 168 cm, weight: 61.2 kg). Abdominal CT showed a thickened, contrast-enhanced wall of the gallbladder (Fig. 1). Percutaneous transhepatic gallbladder drainage (PTGBD) was performed initially. Her abdominal pain subsided and CRP decreased to 1.33 mg/dl after the PTGBD. After 4 days, laparoscopic cholecystectomy was performed. The gallbladder appeared acutely inflamed and contained no stones. Histopathologic examination of the resected gallbladder showed acute cholecystitis. The patient's postoperative progress was uneventful, and she was discharged 5 days after surgery.
AAC generally occurs in patients after major surgery, in the presence of critical illnesses such as trauma, burns, and sepsis, and in the elderly.1 However there have been recent reports of acalculous cholecystitis in young healthy patients who had none of the established risk factors.6,8 The patient described in this case report was young and healthy and exhibited none of the standard risk factors other than recent surgery, although laparoscopic appendectomy is not considered to be a major operation.
The pathogenesis of AAC in patients with no risk factors is as yet unclear. The commonest postulated pathogenesis of AAC is bile stasis resulting in a change of bile composition and ischemia.2 Factors known to contribute to bile stasis in the postoperative patient are fasting, anesthesia, dehydration, fever, and narcotics for the relief of pain.9 I believe that the most probable mechanism of AAC in this woman was bile stasis that resulted from the anesthesia given to her during the appendectomy and the narcotics given for the relief of pain.
AAC is difficult to diagnose because clinical signs such as abdominal pain and fever, and laboratory test results are non-specific. Moreover, these findings may be masked in postoperative patients. Delayed diagnosis of AAC is associated with high morbidity and mortality due to the high prevalence of gangrene and perforation.10 Early diagnosis and appropriate treatment can improve outcomes in patients with AAC.11
The treatment options for AAC are cholecystostomy and/or cholecystectomy.9,12 Cholecystectomy generally is considered the definitive therapy, and percutaneous cholecystostomy can be performed safely and rapidly.9 Standard treatment methods of AAC have not yet been established, but it is usually determined by the patient's condition. I believe that early cholecystectomy in this case was an effective treatment tool for avoiding potential failure of conservative management and for preventing recurrence, although the patient was young and had none of the risk factors.
As mentioned above, laparoscopic appendectomy may mask clinical signs and symptoms of AAC. Therefore, when right upper quadrant pain, fever, leukocytosis, and abnormal liver function tests are observed after surgery, physicians should consider the possibility of AAC, and promptly check radiological findings.
AAC arising as a complication of laparoscopic appendectomy has been reported rarely, especially in young healthy patients. When abdominal pain, leukocytosis, fever and abnormal results of liver function tests are observed in patients after appendectomy, the possibility of AAC should be considered. Prompt recognition and appropriate treatment of AAC is necessary to minimize the associated morbidity and mortality.
References
1. Barie PS, Eachempati SR. Acute acalculous cholecystitis. Curr Gastroenterol Rep. 2003; 5:302–309. PMID: 12864960.

2. Huffman JL, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol. 2010; 8:15–22. PMID: 19747982.

3. Kalliafas S, Ziegler DW, Flancbaum L, et al. Acute acalculous cholecystitis: incidence, risk factors, diagnosis, and outcome. Am Surg. 1998; 64:471–475. PMID: 9585788.
4. Awori KO, Hassan SH, Kiptoon DK. Acute acalculous cholecystitis in an outpatient Setting. East Cent Afr J Surg. 2006; 11:48–53.
5. Ryu JK, Ryu KH, Kim KH. Clinical features of acute acalculous cholecystitis. J Clin Gastroenterol. 2003; 36:166–169. PMID: 12544202.

6. Savoca PE, Longo WE, Zucker KA, et al. The increasing prevalence of acalculous cholecystitis in outpatients. Results of a 7-year study. Ann Surg. 1990; 211:433–437. PMID: 2322038.
7. Hui CK. Acute acalculous cholecystitis after laparoscopic appendicectomy that responded to conservative management. Malays J Med Sci. 2011; 18:76–78. PMID: 22135578.
8. Parithivel VS, Gerst PH, Banerjee S, et al. Acute acalculous cholecystitis in young patients without predisposing factors. Am Surg. 1999; 65:366–368. PMID: 10190365.
9. Ottinger LW. Acute cholecystitis as a postoperative complication. Ann Surg. 1976; 184:162–165. PMID: 952563.

10. Barie PS, Fischer E. Acute acalculous cholecystitis. J Am Coll Surg. 1995; 180:232–244. PMID: 7850064.

11. Ganpathi IS, Diddapur RK, Eugene H, et al. Acute acalculous cholecystitis: challenging the myths. HPB (Oxford). 2007; 9:131–134. PMID: 18333128.

12. Kim JY, Lee MK, Kang YJ, et al. Clinical analysis of acalculous cholecystitis. Korean J Hepatobiliary Pancreat Surg. 2005; 9:216–220.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download