Abstract
Backgrounds/Aims
It has been reported that functional hepatogenic differentiation has the possibility to occur in subcutaneous adipose tissue-derived stem cells. However, no studies have investigated whether the adipose tissue-driven stem cells present in various body parts differ according to hepatogenic differentiations. In this study, stem cells were separated from body visceral fat and abdominal subcutaneous adipose tissue, and cultured, and then hepatogenic differentiation was induced. We aim to investigate the possibilities and aspects of hepatogenic differentiations within the two types of fat cells.
Methods
Omental fat tissues were obtained as visceral fat and abdominal subcutaneous adipose tissues were obtained from patients who had suction-assisted lipectomy. Stem cells were separated from the obtained fat tissues, and then, hepatogenic differentiation was carried out by utilizing 2-step differentiation protocols.
Results
After the differentiation, two types of cultured cells that showed the similar neuron-like shapes were changed to cuboidal shapes and included several binucleated cells which could be characteristics of mature hepatocytes. We confirmed that hepatocyte specific genes and proteins such as albumin and CYP3A4 were being expressed. By utilizing the ELISA test, we were able to observe that the albumin was secreted into the culture fluids in both cells. After completing the differentiation, we observed the presence of the hepatocyte specific properties by confirming glycogen storage within the cells and the ICG reagent uptake.
A liver transplantation would be the only definite treatment of liver failure at the last stage. However, a lot of patients die without having the transplantations due to the lack of people who provide livers as compared to the patients who are in need of the liver transplantations.
As recent emerging of the regenerative medicine, the hepatocyte transplantation gained attentions as replacements of the liver transplants for patients with the last stage of liver failures. It was reported in several researches that hepatocyte transplantation lowered the mortality rates of the patients with acute liver failures and would be helpful in treatment of the patients with metabolic liver diseases.1-3 However, the challenges of supplying healthy and sufficient amounts of hepatocytes, are still unsolved. A stem cell with the possibility of self-replications and differentiates to various cells is being raised as a challenging solution. Among several stem cells, mesenchymal stem cells (MSC), which are adult stem cells, have many advantages in terms of cell usage for experiments. Such efficiency, stability, and possibility of excluding ethical issues and immunological rejection responses have a lower differentiation capacity than the embryonic stem cells.4,5 The mesenchymal stem cells were not only found in bone marrows of the body but also in several connective tissues and peripheral blood vessels. Among those, adipose tissue-derived mesenchymal stem cell (ADSC) has gained interests as it is easy to absorb large quantities as compared to other tissues in the body.6-8
So far, it has been reported that hepatogenic differentiation is possible to occur in the stem cells derived from subcutaneous adipose tissues.3,9-12 However, no studies have indicated that the adipose tissue-derived stem cells presented in various body parts have hepatogenic differentiations. In this study, the stem cells were separated from body visceral fat, and cultured, and then, hepatogenic differentiation was carried out. Thus, we examined whether the stem cells, as obtained from the visceral fat except for subcutaneous adipose tissues, were differentiated from mature hepatocytes.
For visceral fat, omental tissue was obtained from patients who voluntarily agreed with the experiment among those who had abdominal surgery. Abdominal subcutaneous adipose tissue was separated from the patients who had suction-assisted lipectomy in plastic surgery hospitals. This study was carried out with the approval of Bioethics Committee in Catholic University of Korea.
The collected fat tissues were washed with PBS solutions (Gibco BRL, Grand Island, NY, USA) and cut into smaller pieces. Then 25 ml of the pieces were placed in sterilized 50 ml conical tubes and type I collagenase (Gibco BRL), with 0.075%, was added and incubated at 37℃ for 30 minutes in a shaking incubator in order to eliminate other substrates besides the fat tissues. Then, the type I collagenase was neutralized by Dulbecco's modified Eagle's medium-low glucose (DMEM-LG, Gibco BRL) containing 10% fetal bovine serum (FBS, Gibco BRL) and the samples were centrifuged at 3,000G for 5 minutes. The precipitates were separated by eliminating the supernatants, which were the fat tissue layers on top. The precipitates were washed with DMEM-LG several times, then placed in a T75 flask with DMEM medium containing 10% FBS/antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin), and incubated in an incubator at 37℃ with 5% CO2. The medium was replaced twice a week and if confluent of the cultured cells were 70-80% of the culture plate, the cells were subcultured twice using the 0.125% trypsin-EDTA (Gibco BRL).
Before inducing the hepatogenic differentiation of the 1st or 2nd subcultured ADSC, plasma was eliminated via conditioning for 2 days in order to stop cell proliferations, and the samples were cultured in DMEM medium containing 20 ng/ml of hepatocyte growth factor (HGF, SIGMA-ALDRICH) and 10 ng/ml of fibroblast growth factor-basic (bFGF, Gibco BRL). Then, hepatogenic differentiation utilizing 2-step protocol was induced for 3 weeks. In the first step, the differentiation was carried out by culturing for 7 days in DMEM medium containing 20 ng/ml of HGF, 10 ng/ml of bFGF and 4.9 mmol/L of nicotinamide. In the second step, the cells were differentiated and matured by culturing for 2 weeks in DMEM medium containing 20 ng/ml of oncostatin M (OSM, SIGMA-ALDRICH), 1 µmol/L of dexamethasone and 10 µl/ml of ITS Premix (100 µmol/L insulin+6.25 µg/ml transferin+3.6 µmol/L selenious acid+1.25 mg/ml BSA +190 µmol/L linoleic acid, BD Biosciences). The medium was replaced twice a week and cell morphology was examined in each step with an optic microscope.
RNAs of the whole cells used in the experiment were separated from the cells using trizol reagent. Polymerase chain reaction was performed using the superscript III reverse transcripase (Invitrogen, Tokyo, Japan) and the hepatocyte specific gene primer was used in order to examine the differentiated hepatocytes. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control.
In order to measure the albumin that was secreted to the medium, the cultured cells incubated in the plasma removed the DMEM-LG medium by blocking the supply of nutrients for 20 hours. Then, the albumin secretions, both before and after the differentiation, were analyzed utilizing the human albumin ELISA kit.
In order to confirm the pigmentation of glycogen molecules within cells, the cells were washed with PBS 3 times and treated with 4% paraformaldehyde for 1 hour. After oxidizing with 1% periodic acid at room temperature for 20 minutes, the samples were treated with Schiff's reagent for 40 minutes and counterstained using haematoxylin. We examined whether the purple-stained glycogens were present or not by using an optic microscope.
Both ADSC obtained from omental fat and subcutaneous adipose tissues showed similar neuron-like morphology. When the hepatogenic differentiation was completed, although some of the cells were separated on the culture plate, both cells had a cuboidal shape, and several binucleated cells which were exhibited, could be a characteristic of mature hepatocytes (Fig. 1).
Before the differentiation steps, ADSC obtained from omental fat and subcutaneous adipose tissues were expressed weakly or not expressed with regards to ALB and CYP3A4, the hepatocyte specific genes. As the differentiation proceeded, the expression gradually increased (Fig. 2).
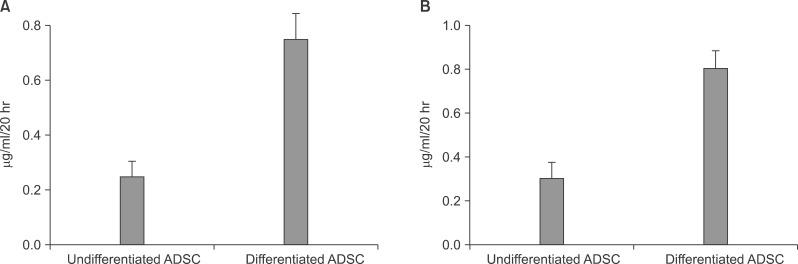
After the differentiation was completed in ADSC obtained from omental fat and subcutaneous adipose tissues, the albumin secreted in medium that nutrients were not supplied for 20 hour by eliminating the plasma was measured. As a result, the ADSC of the subcutaneous adipose tissue had 25±0.24 µg/ml/20 hr in the undifferentiated cells and 0.75±0.24 µg/ml/20 hr in the differentiated cells. The ADSC of the omental fat had 0.30±0.21 µg/ml/20 hr in the undifferentiated cells and 0.80±0.14 µg/ml/20 hr in the differentiated cells (Fig. 3).
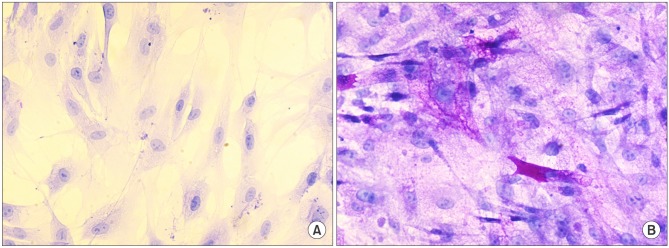
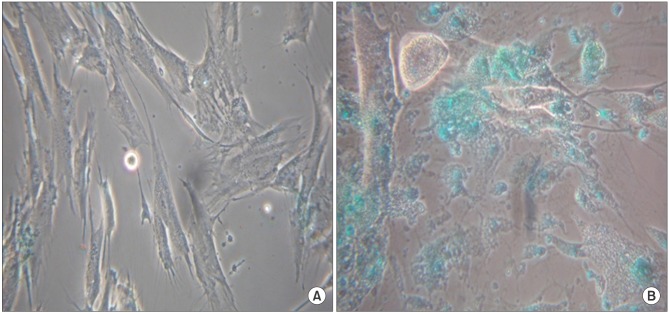
Before the differentiation steps, the ADSC obtained from omental fat indicated negative PAS. However, after the differentiation steps, we observed that most of the cytoplasm insides were stained with intense purple (Fig. 4).
Before the differentiation steps, the ADSC obtained from omental fat did not show the ICG cellular uptakes. However, after the differentiation steps, we observed that most of the cytoplasm insides were green due to the ICG cellular uptakes (Fig. 5).
In this study, we separated ADSC from subcutaneous adipose tissues and omental fat tissues, and then, hepatogenic differentiation was induced. Whereas in previous studies reported, with regards to the hepatogenic differentiations using abdomen or thigh subcutaneous adipose tissues, we focused on intraperitoneal visceral fats and aimed to examine the hepatogenic differentiations of MSC obtained from the intraperitoneal visceral fats.
This study can be used as a clinical trial. When the patient plans for a major hepatectomy is faced with a liver failure risk after the surgery, this study can perform post cellular treatments by securing the omental fat tissues during the surgery.
The same method of hepatogenic differentiation of bone marrow-derived stem cell (BMSC) was used to determine the external factors and the injection times used in the differentiation of this study.13 Undifferentiated ADSC in both subcutaneous adipose tissues and omental fat tissues had similar neuron-like shapes, and in the 3rd week of the differentiation induction, most of the ADSC had a cuboidal shape and indicated mature hepatocyte morphologic characteristics including several binucleated cells. The RT-PCR, carried out ALB and CYP3A4 in order to investigate whether the hepatocyte specific genes were expressed or not, resulted in weak or negative gene expressions for both undifferentiated ADSC, and then, the ALB and CYP3A4 were expressed upon the completion of the differentiation.
Lee et al.13 performed that hepatogenic differentiations were induced in bone marrow-derived mesenchymal stem cells, and then, glycogen storage capacity and urea formation capacity in the differentiated cells were evaluated thereby proving that the differentiated cells indicated a unique property of the hepatocytes. This study confirmed that the differentiated cells show the unique properties of the hepatocytes by confirming the ICG uptakes in cytoplasm and detecting the albumin secreted outside the cells besides the glycogen storage capacity.
Albumin is one of the most abundant proteins among the substances synthesized in the hepatocytes. Although albumin can be detected in early stage of liver development, most of them are detected in the mature hepatocytes. In order to confirm this, the albumin secreted outside the cells was measured using ELISA test. As a result, the albumin secretion in both undifferentiated ADSC was trivial, and then, a large quantity of albumin was secreted after the differentiation. Banas et al.14 also measured the quantity of albumin secreted outside the cells in order to confirm the properties of differentiated hepatocytes. The results showed the hepatocytes that were differentiated for 50 days had about 3 times more albumin secretions than the undifferentiated hepatocytes which were similar to the results of the current study.
Body adipose tissues have many advantages: abundant, easily separated without giving huge risks to patients, and possibilities to regenerate. Multipotent mesenchymal stem cells are also present in the adipose tissues and the quantitative limitation of adult stem cells can be compensated by using the multipotent mesenchymal stem cells.7,8 The ADSC is known to have similar differentiation and proliferation characteristics with BMSC, the representative mesenchymal stem cell as previously reported. However, in terms of cell yields, BMSC is only 0.01% of the bone marrow nucleated cells but approximately 100,000 mesenchymal stem cells per gram are present in an adipose tissue and can proliferate in large quantities when utilized as an ideal stem cell source.
In the case of BMSC, collecting the stem cells possibly gives risks of severe pains and complications to patients. In case of the ADSC, however, cell collecting is very easy and simple, and thereby, more useful.15 ADSC is basically differentiated not only in mesenchymal cells such as fat cells, myocytes, osteocytes, and chondrocytes etc but also in non-mesenchymal cells such as nerve cells, pancreas endocrine cells, hepatocytes, vascular endothelial cells, and cardiomyocytes.4,16-21 Especially, the hepatogenic differentiation of ADSC was reported for the first time by Seo et al.11 in 2005 and the differentiation procedures which took 3-4 weeks were introduced. Taléns-Visconti et al.12 compared the hepatogenic differentiations of BMSC and ADSC. Stem cells were obtained from cancellous bones in patients who had hip joint arthroplasty and the tissues were obtained by abdominal suction-assisted lipectomy. Then hepatogenic differentiation was induced. Accordingly, it was proved that ADSC had similar hepatogenic differentiation with BMSC and had better proliferation capacity even though the culturing took longer. More than 3 weeks of differentiation time were required for the current study, and since that the differentiation took a relatively long time, a concern was that the large portion of cells were necrotized. This might be because either the plasma was removed in order to stop the proliferation of the cells and induce the differentiation or only a low level of glucose is being supplied. To deal with such concerns, appropriate cell density would be maintained and other methods should be applied to shorten the differentiation times. Recently, Banas et al.3 successfully decreased the differentiation time, which normally takes more than 3 weeks, for the hepatocytes originated from ADSC to only 13 days. The authors applied hepatic damages to rats and observed their recovery from damaged liver functions, and thereby, providing the possible cell therapy products for the liver diseases.
In conclusion, it was confirmed that ADSC, isolated from visceral fat tissues, were able to differentiate the cells with mature hepatocytes shapes as well as hepatocytes specific functions. It was also shown that omental fat tissues, which can be easily obtained via abdominal surgery, except for the subcutaneous adipose tissues, could be differentiated hepatogenically; these cells would be good resources of cellular treatment for liver diseases in the future.
References
1. Aurich I, Mueller LP, Aurich H, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007; 56:405–415. PMID: 16928726.

2. Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009; 58:570–581. PMID: 19022918.

3. Banas A, Teratani T, Yamamoto Y, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009; 24:70–77. PMID: 18624899.

4. Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005; 87:125–128. PMID: 15733747.

5. Rooney GE, Nistor GI, Barry FB, et al. In vitro differentiation potential of human embryonic versus adult stem cells. Regen Med. 2010; 5:365–379. PMID: 20455648.
6. Young HE, Mancini ML, Wright RP, et al. Mesenchymal stem cells reside within the connective tissues of many organs. Dev Dyn. 1995; 202:137–144. PMID: 7734732.

7. Meliga E, Strem BM, Duckers HJ, et al. Adipose-derived cells. Cell Transplant. 2007; 16:963–970. PMID: 18293895.

8. Nakagami H, Morishita R, Maeda K, et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006; 13:77–81. PMID: 16733294.

9. Banas A, Teratani T, Yamamoto Y, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008; 26:2705–2712. PMID: 18535155.

10. Coradeghini R, Guida C, Scanarotti C, et al. A comparative study of proliferation and hepatic differentiation of human adipose-derived stem cells. Cells Tissues Organs. 2010; 191:466–477. PMID: 20051678.

11. Seo MJ, Suh SY, Bae YC, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005; 328:258–264. PMID: 15670778.

12. Taléns-Visconti R, Bonora A, Jover R, et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006; 12:5834–5845. PMID: 17007050.

13. Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004; 40:1275–1284. PMID: 15562440.

14. Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007; 46:219–228. PMID: 17596885.

15. Sen A, Lea-Currie YR, Sujkowska D, et al. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J Cell Biochem. 2001; 81:312–319. PMID: 11241671.

16. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7:211–228. PMID: 11304456.

17. Xu Y, Malladi P, Wagner DR, et al. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Curr Opin Mol Ther. 2005; 7:300–305. PMID: 16121695.
18. Wosnitza M, Hemmrich K, Groger A, et al. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007; 75:12–23. PMID: 17244018.

19. Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002; 294:371–379. PMID: 12051722.

20. Erickson GR, Gimble JM, Franklin DM, et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002; 290:763–769. PMID: 11785965.

21. Fraser JK, Schreiber R, Strem B, et al. Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin Pract Cardiovasc Med. 2006; 3(Suppl 1):S33–S37. PMID: 16501628.

Fig. 1
Morphology of MSC from abdominal subcutaneous adipose tissue and omental fat during the differentiation protocol. Before differentiation culture, MSC from abdominal subcutaneous adipose tissue (A) and omental fat (B) showed bipolar neuron-like morphology. However, both types of MSC from abdominal subcutaneous adipose tissue (C) and omental fat (D) significantly changed the morphology, and developed a cuboidal shape and many of them appeared to be binucleated cells (arrows), which are typical hepatocytes after differentiation steps (magnification: A, B ×100; C, D ×200).
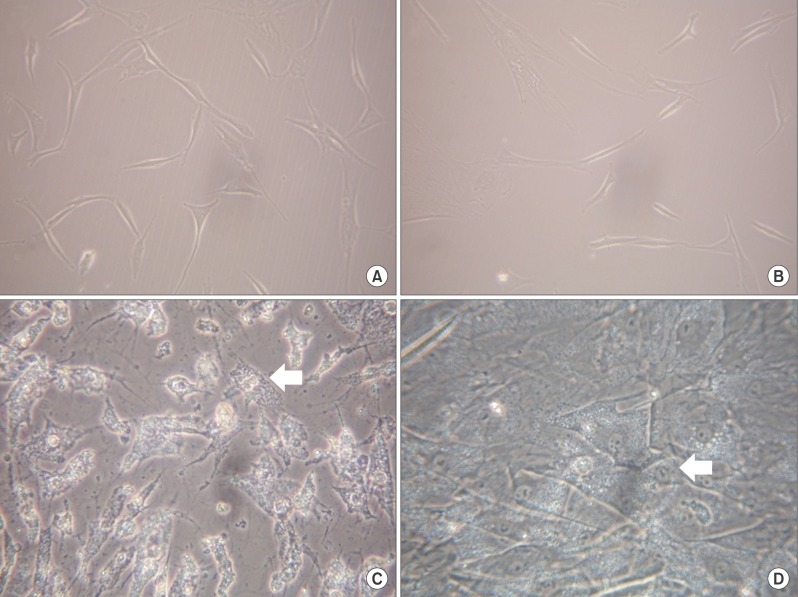
Fig. 2
RT-PCR analysis of the expressions of mRNA for ALB and CYP3A4 (compared with that of the human hepatoma cells, Hep G2). The expression of ALB in MSC from abdominal subcutaneous adipose tissue (black bars) and in MSC from omental fat (white bars) were detected at all time points and increased with differentiation time (A). The expression of CYP3A4 was detected at 2 weeks post-induction and then increased with differentiation time (B).
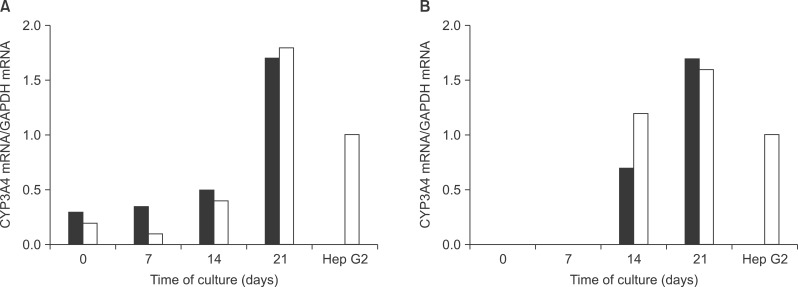
Fig. 3
Albumin secretion by both types of ADSC from abdominal subcutaneous adipose tissue (A) and omental fat (B). The amount of albumin released into the medium before and after hepatic differentiation in vitro analyzed by ELISA is shown. Data are expressed as mean±SEM of three independent experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download