This article has been
cited by other articles in ScienceCentral.
Abstract
Bronchobiliary fistula (BBF) is a rare complication of radiofrequency ablation (RFA), in which there is abnormal communications between the biliary tract and the bronchial trees. Surgery should only be considered for BBF when non-invasive interventions have failed. In this report, we describe the surgical management for BBF when complicated by an abscess that was encountered after RFA in a 52-year-old woman with recurrent hepatocellular carcinoma (HCC). She had previously undergone central bisectionectomy of HCC 7 years ago, and had been treated with a sixth transarterial chemoembolization and first RFA for recurrent HCC after the operation. After the liver abscess and BBF occurred in the posterior section of the liver, she received posterior sectionectomy and hepaticojejunostomy, drainage of the lung abscess, diaphragmatic resection and repair because it was impossible to drain the abscess radiologically. Symptomatic improvements were being achieved through operative treatments where pleural effusion and pneumonic consolidation was obliterated on a 2-months follow-up image.
Go to :

Keywords: Bronchobiliary fistula, Radiofrequency catheter ablation, Hepatocellular carcinoma, Hepatectomy
IINTRODUCTION
Bronchobiliary fistula (BBF), an uncommon complication of radiofrequency ablation (RFA), is due to abnormal communications between the biliary tract and bronchial trees. In addition, it may be presented as a complication of either hepatic abscess, hydatid disease of the liver, hepatic malignancy, trauma, or liver surgery.
1,
2 The optimal management of BBF is still controversial and is often associated with high morbidity and mortality rates. Although BBF has traditionally been treated surgically, nonsurgical interventional management such as endoscopic drainage or percutaneous abscess drainage have recently been suggested as the first therapeutic option.
3 If the nonsurgical treatment of BBF fails, surgical exploration is inevitable. Herein, we describe a case of BBF due to RFA for recurring hepatocellular carcinoma which was successfully treated with surgical resection and drainage.
Go to :

CASE
A 52-year-old woman was treated with percutaneous RFA for recurrent hepatocellular carcinoma (HCC) at segment 7. She had been diagnosed with hepatitis B in 1997. Her past medical history revealed that she had undergone central bisectionectomy for a 5-cm HCC, which was located between segments 4 and 8, seven years ago. From August 2007 to May 2009, after the operation, she had been treated with transarterial chemoembolization for a total of six times due to a recurrent HCC at her remnant liver segments. When the seventh recurrence was detected by follow-up liver computed tomography (CT), RFA was performed percutaneously under ultrasonographic guidance in the 3.5-cm viable tumor of segment 7, located in the liver dome and close to the diaphragm. After RFA, the total bilirubin level gradually increased without any other symptoms, and a level of 10.9 mg/dl was measured 5 days post-RFA. A liver CT was performed thereafter, and showed obstructions of the entire right portal flow with right bile duct dilatation and decreased perfusions of the atrophic right posterior section. Endoscopic retrograde cholangiopancreatography (ERCP) was performed and showed structure of the common hepatic ducts and dilatation of both intrahepatic ducts (
Fig. 1). Therefore, endoscopic retrograde biliary drainage (ERBD) was performed, and two plastic stents (pigtail stents with 7-french and 10-cm length) were inserted in the right and left bile ducts, respectively. The decreased level of total bilirubin was confirmed three days after stent insertions, and the patient was discharged without symptoms on that same day. The patient was then admitted through the emergency room 25 days later due to high fever (38.3℃) with no other symptoms. Abdominal CT showed that the RFA zone had changed to an abscess pocket with small air bubbles. The patient was empirically treated with antibiotics for ten days and discharged with mild coughing according to the decreasing abscess cavities.
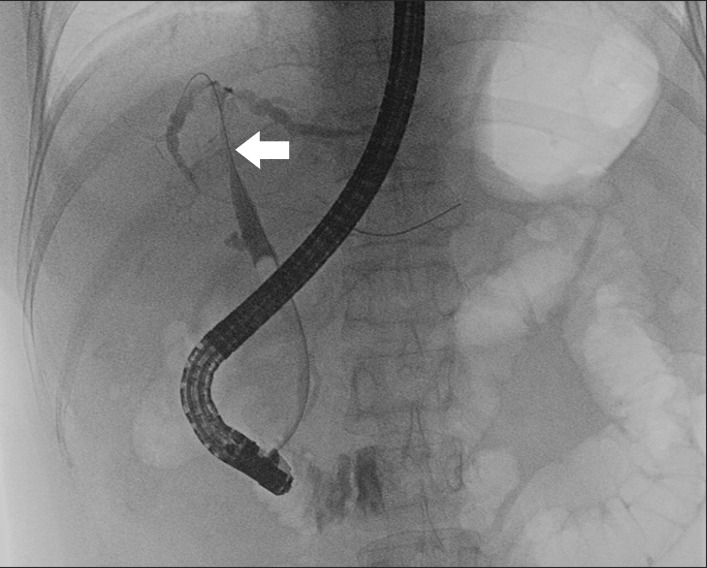 | Fig. 1Endoscopic retrograde cholangiopancreatography shows structure of the common hepatic duct (arrow) and mild dilatation of the left and right hepatic ducts. 
|
The patient revisited the emergency room two days after discharge, i.e., 41 days after the ERBD was performed, presenting with a persistent cough producing yellow-green sputum and dyspnea with high-grade fever of more than 38℃. Coughing increased when lying down and improved when sitting upright. It was accompanied by shortness of breath and right-sided pleuritic chest pains. Blood test exhibited mild liver dysfunction, inflammatory reaction (white blood cell counts 23.1×1,000/µl, normal range, 3.2-8.6×1,000/µl; C-reactive protein 12.2 mg/dl, normal range ≤0.3 mg/dl), and hypoxemia, where the sputum analysis revealed a total bilirubin level of 3.1 mg/dl. A chest X-ray and abdominal CT demonstrated localized pneumonia in the lower right lobe of the lung and appeared as a consolidation. An abscess pocket in the right basal lung showed a fistula from an abscess cavity in the liver to the lower right lobe of the lung (
Fig. 2).
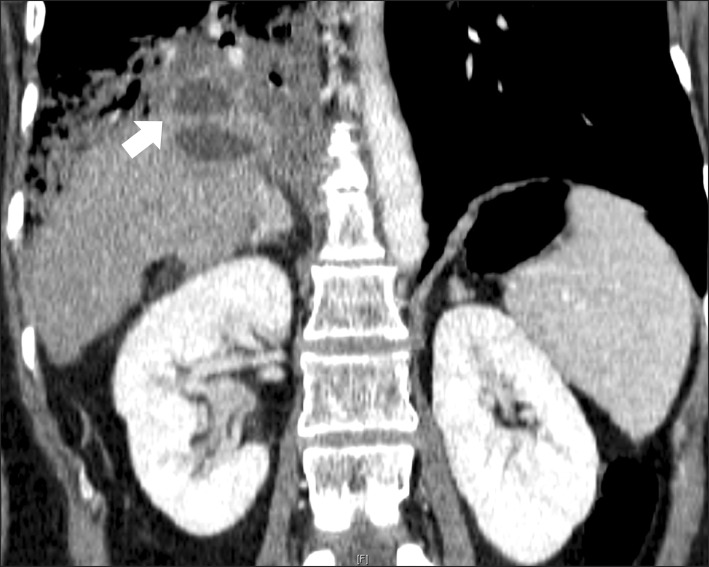 | Fig. 2Abdominal CT reveals another newly appeared abscess pocket in the right basal lung and a fistula (arrow) showing communications between the radiofrequency-ablated cavity in the liver and the bronchial tree, including the pneumonic consolidation involving the middle right and lower lobes of the lung. 
|
Surgical treatment was determined because the patient gradually aggravated with uncontrolled septic shock, and it was impossible to access the abscess pocket for drainage percutaneously or endoscopically due to the location. Right posterior sectionectomy of the liver, a combined resection of the diaphragm and drainage of lung abscess with tube thoracostomy were all performed. The abscess cavity of the right posterior section contained bile-stained necrotic materials, and was communicated with an abscess in the right basal lung through a pinpoint perforation of the inflamed diaphragm. The infected tissues in the dome were debrided. Apart from localized visceral pleural damages, the lower right lobe appeared to be healthy and capable of full expansion. Thus, resection was not performed. The diaphragmatic defect measured 10 cm in the largest diameter and closed primarily around the muscle. The right and left bile ducts around an abscess of the RFA zone were disrupted, and thus, a biliary reconstruction to the left lateral section by Roux-en-Y hepaticojejunostomy was required (
Fig. 3). Previously inserted ERBD stents were all removed before the biliary reconstruction. The operative time was 460 minutes, and the amount of blood loss was 760 ml. The chest drain and subdiaphragmatic drain were removed on the seventh postoperative day. No postoperative complications occurred, and nine days after surgery, the patient was discharged with symptomatic improvements. A 2-months follow-up chest X-ray demonstrated that the pleural effusion and pneumonic consolidation in the right basal lung were obliterated (
Fig. 4). Clinical follow-ups continued for eight months without any further complaints.
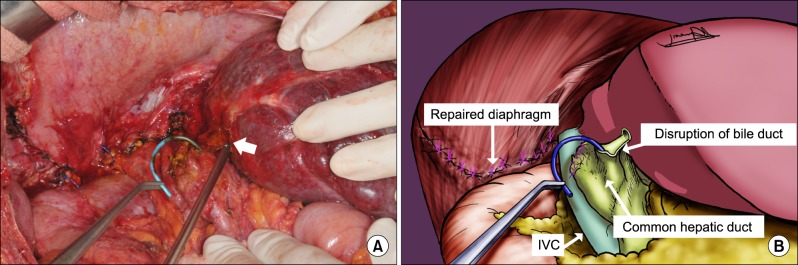 | Fig. 3(A) Operative field after right posterior sectionectomy and primary repair of the diaphragmatic defect shows exposure of left biliary stent (clamping with curved forceps) and opening of the left hepatic duct (arrow). (B) Schematic figure. 
|
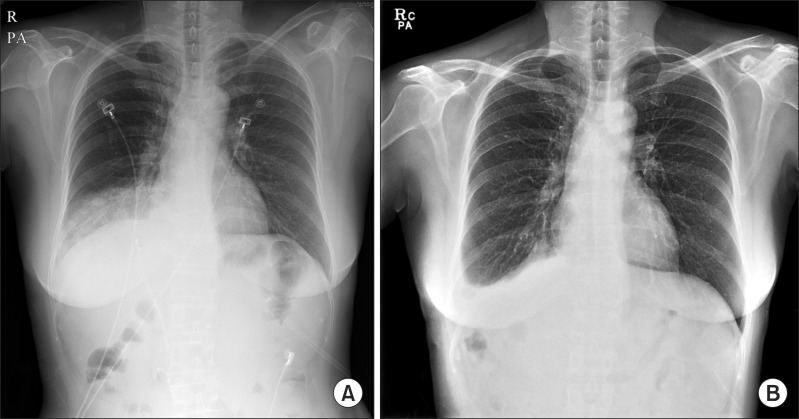 | Fig. 4(A) Preoperative chest X-ray shows pleural effusion and pneumonic consolidation in the lower right lobe of the lung. (B) A follow-up chest X-ray taken 2 months after operation demonstrates improvements of pneumonia and pleural effusion in the right basal lung. 
|
Go to :

DISCUSSION
BBF was first reported by Peacock in a patient with hydatid disease of the liver.
4 RFA is one of a variety of causes for acquired BBF. In hepatocellular carcinoma, the use of RFA increased as an alternative or adjuvant treatment of surgical resection. As a result, the incidence of complications associated with RFA has also increased. The number of reported cases on BBF associated with RFA has recently increased.
Pathogenesis of RFA-related BBF probably results from diaphragmatic thermal injuries and intrahepatic biloma formations. In our patients, these causes seem to be the main factors for the development of the BBF. The developmental steps of the BBF occurring after RFA can be assumed as follows: collateral thermal injury of the diaphragm; biloma formation around the ablative zone; development of diaphragmatic defect with increased pressures due to a growing biloma; rupture of the biloma into the diaphragmatic defect; development of bilious pleural effusion and basal pneumonia; and formation of a fistula between the diaphragmatic defect and injured terminal bronchioles.
5 Moreover, biliary tract injury during RFA causes biliary stenosis and cholangitis, which consequently requires ERBD for our patients. Even after the biliary decompression was achieved with ERBD, BBF developed gradually due to thermal injuries of the diaphragm.
The typical symptom of BBF is bilioptysis. Bilioptysis defined productive coughs of bile-tinged sputum in nearly all the reported cases.
2 The presence of bile in the sputum can be easily identified by using biochemical analysis of the sputum for bilirubin. Other symptoms and signs include orthopnea, chest pain, fever, jaundice and abdominal pain. Diagnosis can be confirmed by imaging procedures such as the hepatobiliary imino-diacetic (HIDA) scan, percutaneous transhepatic cholangiography (PTC), ERCP, CT or the magnetic resonance cholangiopancreatography (MRCP).
2 In this case, abdominal CT was performed when patients had symptoms such as bilioptysis, and showed fistula tracts between the biliary system and bronchial tree through the diaphragm.
There is still no consensus on the optimal treatments of BBF. Generally, there are two treatment options for BBF, such as operative procedures and nonsurgical interventions. Surgical treatments have traditionally been performed with two-fold principles: first, to relieve any biliary obstructions and permit continuous free drainage of bile into the digestive tract usually through the creation of Roux-en-Y hepaticojejunostomy; and second, to drain all abscesses and to excise the fistula tract together with the diseased lung segment or to completely obliterate the fistula tract.
3,
6 In this case, liver resection was performed due to disruptions of the bile duct wall and liver abscess, and thus, the abscess drainage of the lung was performed without resections of lung parenchyma. However, surgery for BBF in a patient, who has undergone liver resections and who is in a state of sepsis, could add to the negative sentiments for retrieval.
3,
7 Nonsurgical treatments such as radiological and endoscopic interventions, which are less riskier than surgery, have been recently highlighted. In the previous cases, patients with BBF following RFA which are complicated by biloma or liver abscess successfully underwent external drainages with or without endoscopic drainage.
5,
8 There are reports on successful treatments of endoscopic nasobiliary drainage or endoscopic sphincterotomy in all cases.
1 Embolization of the BBF tract using cyanoacrylate glue through bronchoscopy has been reported as an alternative nonsurgical approach.
9 However, these less invasive interventions usually involve long-term drainage maintenances with antibiotics after symptom improvements for complete remissions of the BBF and repeated for radiological manipulations.
3,
10
Although the operative procedure for BBF may be complex with significant morbidity and mortality, surgery is ultimately considered in cases where the nonsurgical interventional techniques have failed or when there are complications from the underlying diseases, similar to our patient who had impossible accesses to the abscess pockets, uncontrolled sepsis, and progressions of pneumonic consolidation. In summary, a definite procedure such as surgery for BBF should be considered aggressively when the non-invasive interventions fail.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download